Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice
- PMID: 18562603
- PMCID: PMC3329761
- DOI: 10.1523/JNEUROSCI.0829-08.2008
Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice
Abstract
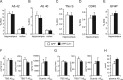
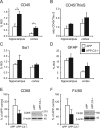
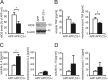
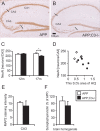
Complement factor C3 is the central component of the complement system and a key inflammatory protein activated in Alzheimer's disease (AD). Previous studies demonstrated that inhibition of C3 by overexpression of soluble complement receptor-related protein y in an AD mouse model led to reduced microgliosis, increased amyloid beta (Abeta) plaque burden, and neurodegeneration. To further address the role of C3 in AD pathology, we generated a complement C3-deficient amyloid precursor protein (APP) transgenic AD mouse model (APP;C3(-/-)). Brains were analyzed at 8, 12, and 17 months of age by immunohistochemical and biochemical methods and compared with age-matched APP transgenic mice. At younger ages (8-12 months), no significant neuropathological differences were observed between the two transgenic lines. In contrast, at 17 months of age, APP;C3(-/-) mice showed significant changes of up to twofold increased total Abeta and fibrillar amyloid plaque burden in midfrontal cortex and hippocampus, which correlated with (1) significantly increased Tris-buffered saline (TBS)-insoluble Abeta(42) levels and reduced TBS-soluble Abeta(42) and Abeta(40) levels in brain homogenates, (2) a trend for increased Abeta levels in the plasma, (3) a significant loss of neuronal-specific nuclear protein-positive neurons in the hippocampus, and (4) differential activation of microglia toward a more alternative phenotype (e.g., significantly increased CD45-positive microglia, increased brain levels of interleukins 4 and 10, and reduced levels of CD68, F4/80, inducible nitric oxide synthase, and tumor necrosis factor). Our results suggest a beneficial role for complement C3 in plaque clearance and neuronal health as well as in modulation of the microglia phenotype.
Figures



Similar articles
-
Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice.Sci Transl Med. 2017 May 31;9(392):eaaf6295. doi: 10.1126/scitranslmed.aaf6295. Sci Transl Med. 2017. PMID: 28566429 Free PMC article.
-
Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer's disease.J Neurochem. 2008 Sep;106(5):2080-92. doi: 10.1111/j.1471-4159.2008.05558.x. Epub 2008 Jul 9. J Neurochem. 2008. PMID: 18624920 Free PMC article.
-
Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer's Disease.J Neurosci. 2016 Jan 13;36(2):577-89. doi: 10.1523/JNEUROSCI.2117-15.2016. J Neurosci. 2016. PMID: 26758846 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
Cited by
-
Deficiency of terminal complement pathway inhibitor promotes neuronal tau pathology and degeneration in mice.J Neuroinflammation. 2012 Sep 18;9:220. doi: 10.1186/1742-2094-9-220. J Neuroinflammation. 2012. PMID: 22989354 Free PMC article.
-
Ablation of CCAAT/Enhancer-Binding Protein Delta (C/EBPD): Increased Plaque Burden in a Murine Alzheimer's Disease Model.PLoS One. 2015 Jul 31;10(7):e0134228. doi: 10.1371/journal.pone.0134228. eCollection 2015. PLoS One. 2015. PMID: 26230261 Free PMC article.
-
Mouse brain transcriptome responses to inhaled nanoparticulate matter differed by sex and APOE in Nrf2-Nfkb interactions.Elife. 2020 Jun 24;9:e54822. doi: 10.7554/eLife.54822. Elife. 2020. PMID: 32579111 Free PMC article.
-
Microglia in Alzheimer's Disease: a Key Player in the Transition Between Homeostasis and Pathogenesis.Neurotherapeutics. 2022 Jan;19(1):186-208. doi: 10.1007/s13311-021-01179-3. Epub 2022 Mar 14. Neurotherapeutics. 2022. PMID: 35286658 Free PMC article. Review.
-
The Complement System in the Central Nervous System: From Neurodevelopment to Neurodegeneration.Biomolecules. 2022 Feb 21;12(2):337. doi: 10.3390/biom12020337. Biomolecules. 2022. PMID: 35204837 Free PMC article. Review.
References
-
- Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–294. - PubMed
-
- Eikelenboom P, Hack CE, Rozemuller JM, Stam FC. Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;56:259–262. - PubMed
-
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
