Preferential reactivation of motivationally relevant information in the ventral striatum
- PMID: 18562607
- PMCID: PMC3844781
- DOI: 10.1523/JNEUROSCI.1054-08.2008
Preferential reactivation of motivationally relevant information in the ventral striatum
Abstract
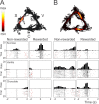
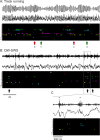
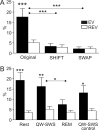
Spontaneous "off-line" reactivation of neuronal activity patterns may contribute to the consolidation of memory traces. The ventral striatum exhibits reactivation and has been implicated in the processing of motivational information. It is unknown, however, whether reactivating neuronal ensembles specifically recapitulate information relating to rewards that were encountered during wakefulness. We demonstrate a prolonged reactivation in rat ventral striatum during quiet wakefulness and slow-wave but not rapid eye movement sleep. Reactivation of reward-related information processed in this structure was particularly prominent, and this was primarily attributable to spike trains temporally linked to reward sites. It was accounted for by small, strongly correlated subgroups in recorded cell assemblies and can thus be characterized as a sparse phenomenon. Our results indicate that reactivated memory traces may not only comprise feature- and context-specific information but also contain a value component.
Figures


Comment in
-
Reactivation in ventral striatum during hippocampal ripples: evidence for the binding of reward and spatial memories?J Neurosci. 2008 Oct 1;28(40):9895-7. doi: 10.1523/JNEUROSCI.3778-08.2008. J Neurosci. 2008. PMID: 18829947 Free PMC article. Review. No abstract available.
References
-
- Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. - PubMed
-
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
