Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory
- PMID: 18562617
- PMCID: PMC6670907
- DOI: 10.1523/JNEUROSCI.0412-08.2008
Inducible cAMP early repressor acts as a negative regulator for kindling epileptogenesis and long-term fear memory
Abstract
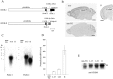
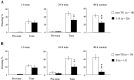
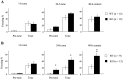
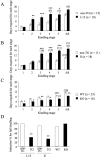
Long-lasting neuronal plasticity as well as long-term memory (LTM) requires de novo synthesis of proteins through dynamic regulation of gene expression. cAMP-responsive element (CRE)-mediated gene transcription occurs in an activity-dependent manner and plays a pivotal role in neuronal plasticity and LTM in a variety of species. To study the physiological role of inducible cAMP early repressor (ICER), a CRE-mediated gene transcription repressor, in neuronal plasticity and LTM, we generated two types of ICER mutant mice: ICER-overexpressing (OE) mice and ICER-specific knock-out (KO) mice. Both ICER-OE and ICER-KO mice show no apparent abnormalities in their development and reproduction. A comprehensive battery of behavioral tests revealed no robust changes in locomotor activity, sensory and motor functions, and emotional responses in the mutant mice. However, long-term conditioned fear memory was attenuated in ICER-OE mice and enhanced in ICER-KO mice without concurrent changes in short-term fear memory. Furthermore, ICER-OE mice exhibited retardation of kindling development, whereas ICER-KO mice exhibited acceleration of kindling. These results strongly suggest that ICER negatively regulates the neuronal processes required for long-term fear memory and neuronal plasticity underlying kindling epileptogenesis, possibly through suppression of CRE-mediated gene transcription.
Figures

Similar articles
-
Inducible cAMP early repressor (ICER) and brain functions.Mol Neurobiol. 2009 Aug;40(1):73-86. doi: 10.1007/s12035-009-8072-1. Epub 2009 May 13. Mol Neurobiol. 2009. PMID: 19434522 Free PMC article. Review.
-
The role of transcription factors cyclic-AMP responsive element modulator (CREM) and inducible cyclic-AMP early repressor (ICER) in epileptogenesis.Neuroscience. 2008 Mar 27;152(3):829-36. doi: 10.1016/j.neuroscience.2007.10.064. Epub 2008 Jan 17. Neuroscience. 2008. PMID: 18295410 Free PMC article.
-
Negative regulation of TLR responses by the neuropeptide CGRP is mediated by the transcriptional repressor ICER.J Immunol. 2007 Jul 1;179(1):607-15. doi: 10.4049/jimmunol.179.1.607. J Immunol. 2007. PMID: 17579082
-
Inducible cAMP early repressor (ICER) in the nervous system--a transcriptional regulator of neuronal plasticity and programmed cell death.J Neurochem. 2003 Dec;87(6):1313-20. doi: 10.1046/j.1471-4159.2003.02116.x. J Neurochem. 2003. PMID: 14713288 Review.
-
Coupling signalling pathways to transcriptional control: nuclear factors responsive to cAMP.Recent Prog Horm Res. 1997;52:121-39; discussion 139-40. Recent Prog Horm Res. 1997. PMID: 9238850 Review.
Cited by
-
Novel Aspects of cAMP-Response Element Modulator (CREM) Role in Spermatogenesis and Male Fertility.Int J Mol Sci. 2023 Aug 8;24(16):12558. doi: 10.3390/ijms241612558. Int J Mol Sci. 2023. PMID: 37628737 Free PMC article. Review.
-
Distinctive roles for amygdalar CREB in reconsolidation and extinction of fear memory.Learn Mem. 2012 Apr 13;19(5):178-81. doi: 10.1101/lm.025783.112. Learn Mem. 2012. PMID: 22505719 Free PMC article.
-
Inducible cAMP early repressor (ICER) and brain functions.Mol Neurobiol. 2009 Aug;40(1):73-86. doi: 10.1007/s12035-009-8072-1. Epub 2009 May 13. Mol Neurobiol. 2009. PMID: 19434522 Free PMC article. Review.
-
Alteration of epileptogenesis genes.Neurotherapeutics. 2009 Apr;6(2):312-8. doi: 10.1016/j.nurt.2009.01.019. Neurotherapeutics. 2009. PMID: 19332325 Free PMC article. Review.
-
Molecular pathways controlling inhibitory receptor expression.Epilepsia. 2012 Dec;53 Suppl 9(0 9):71-8. doi: 10.1111/epi.12036. Epilepsia. 2012. PMID: 23216580 Free PMC article. Review.
References
-
- Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. - PubMed
-
- Ahmed T, Frey JU. Plasticity-specific phosphorylation of CaMKII, MAP-kinases and CREB during late-LTP in rat hippocampal slices in vitro. Neuropharmacology. 2005;49:477–492. - PubMed
-
- Albensi BC, Oliver DR, Toupin J, Odero G. Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp Neurol. 2007;204:1–13. - PubMed
-
- Bading H. Nuclear calcium-activated gene expression: possible roles in neuronal plasticity and epileptogenesis. Epilepsy Res. 1999;36:225–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
