Role of Dot1 in the response to alkylating DNA damage in Saccharomyces cerevisiae: regulation of DNA damage tolerance by the error-prone polymerases Polzeta/Rev1
- PMID: 18562671
- PMCID: PMC2475726
- DOI: 10.1534/genetics.108.089003
Role of Dot1 in the response to alkylating DNA damage in Saccharomyces cerevisiae: regulation of DNA damage tolerance by the error-prone polymerases Polzeta/Rev1
Abstract
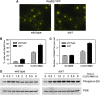
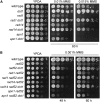
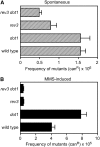
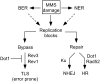
Maintenance of genomic integrity relies on a proper response to DNA injuries integrated by the DNA damage checkpoint; histone modifications play an important role in this response. Dot1 methylates lysine 79 of histone H3. In Saccharomyces cerevisiae, Dot1 is required for the meiotic recombination checkpoint as well as for chromatin silencing and the G(1)/S and intra-S DNA damage checkpoints in vegetative cells. Here, we report the analysis of the function of Dot1 in the response to alkylating damage. Unexpectedly, deletion of DOT1 results in increased resistance to the alkylating agent methyl methanesulfonate (MMS). This phenotype is independent of the dot1 silencing defect and does not result from reduced levels of DNA damage. Deletion of DOT1 partially or totally suppresses the MMS sensitivity of various DNA repair mutants (rad52, rad54, yku80, rad1, rad14, apn1, rad5, rad30). However, the rev1 dot1 and rev3 dot1 mutants show enhanced MMS sensitivity and dot1 does not attenuate the MMS sensitivity of rad52 rev3 or rad52 rev1. In addition, Rev3-dependent MMS-induced mutagenesis is increased in dot1 cells. We propose that Dot1 inhibits translesion synthesis (TLS) by Polzeta/Rev1 and that the MMS resistance observed in the dot1 mutant results from the enhanced TLS activity.
Figures


Similar articles
-
Regulation of tolerance to DNA alkylating damage by Dot1 and Rad53 in Saccharomyces cerevisiae.DNA Repair (Amst). 2010 Oct 5;9(10):1038-49. doi: 10.1016/j.dnarep.2010.07.003. Epub 2010 Jul 31. DNA Repair (Amst). 2010. PMID: 20674515
-
Dot1-dependent histone H3K79 methylation promotes activation of the Mek1 meiotic checkpoint effector kinase by regulating the Hop1 adaptor.PLoS Genet. 2013;9(1):e1003262. doi: 10.1371/journal.pgen.1003262. Epub 2013 Jan 31. PLoS Genet. 2013. PMID: 23382701 Free PMC article.
-
MutSα deficiency increases tolerance to DNA damage in yeast lacking postreplication repair.DNA Repair (Amst). 2020 Jul-Aug;91-92:102870. doi: 10.1016/j.dnarep.2020.102870. Epub 2020 May 21. DNA Repair (Amst). 2020. PMID: 32470850
-
Role of DNA polymerase eta in the bypass of abasic sites in yeast cells.Nucleic Acids Res. 2004 Jul 29;32(13):3984-94. doi: 10.1093/nar/gkh710. Print 2004. Nucleic Acids Res. 2004. PMID: 15284331 Free PMC article.
-
REV1 and DNA polymerase zeta in DNA interstrand crosslink repair.Environ Mol Mutagen. 2012 Dec;53(9):725-40. doi: 10.1002/em.21736. Epub 2012 Oct 13. Environ Mol Mutagen. 2012. PMID: 23065650 Free PMC article. Review.
Cited by
-
Genome Instability Is Promoted by the Chromatin-Binding Protein Spn1 in Saccharomyces cerevisiae.Genetics. 2018 Dec;210(4):1227-1237. doi: 10.1534/genetics.118.301600. Epub 2018 Oct 9. Genetics. 2018. PMID: 30301740 Free PMC article.
-
Therapeutic strategies against hDOT1L as a potential drug target in MLL-rearranged leukemias.Clin Epigenetics. 2020 May 25;12(1):73. doi: 10.1186/s13148-020-00860-2. Clin Epigenetics. 2020. PMID: 32450905 Free PMC article. Review.
-
A Novel Histone Crosstalk Pathway Important for Regulation of UV-Induced DNA Damage Repair in Saccharomyces cerevisiae.Genetics. 2017 Jul;206(3):1389-1402. doi: 10.1534/genetics.116.195735. Epub 2017 May 18. Genetics. 2017. PMID: 28522541 Free PMC article.
-
A DOT1B/Ribonuclease H2 Protein Complex Is Involved in R-Loop Processing, Genomic Integrity, and Antigenic Variation in Trypanosoma brucei.mBio. 2021 Dec 21;12(6):e0135221. doi: 10.1128/mBio.01352-21. Epub 2021 Nov 9. mBio. 2021. PMID: 34749530 Free PMC article.
-
Chromosome-wide histone deacetylation by sirtuins prevents hyperactivation of DNA damage-induced signaling upon replicative stress.Nucleic Acids Res. 2016 Apr 7;44(6):2706-26. doi: 10.1093/nar/gkv1537. Epub 2016 Jan 8. Nucleic Acids Res. 2016. PMID: 26748095 Free PMC article.
References
-
- Bardwell, A. J., L. Bardwell, A. E. Tomkinson and E. C. Friedberg, 1994. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science 265 2082–2085. - PubMed
-
- Boiteux, S., and M. Guillet, 2004. Abasic sites in DNA: repair and biological consequences in Saccharomyces cerevisiae. DNA Repair 3 1–12. - PubMed
-
- Boiteux, S., and M. Guillet, 2006. Use of yeast for detection of endogenous abasic lesions, their source, and their repair. Methods Enzymol. 408 79–91. - PubMed
-
- Bostelman, L. J., A. M. Keller, A. M. Albrecht, A. Arat and J. S. Thompson, 2007. Methylation of histone H3 lysine-79 by Dot1p plays multiple roles in the response to UV damage in Saccharomyces cerevisiae. DNA Repair 6 383–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

