Strategies for the Preparation of Differentially Protected ortho-Prenylated Phenols
- PMID: 18563206
- PMCID: PMC2430722
- DOI: 10.1055/s-2003-36234
Strategies for the Preparation of Differentially Protected ortho-Prenylated Phenols
Abstract
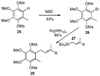
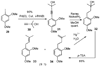
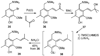
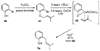
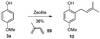
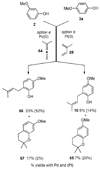
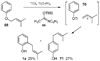
A new process for ortho-prenylation of phenols is presented within the context of known methods. All of the processes are briefly assessed with regards to the substitution patterns and accompanying functional groups tolerated by each strategy. The conclusion reached is that a new procedure using ortho-quinone methides, for which an experimental protocol is provided, offers the greatest generality and flexibility in the preparation of ortho-prenylated phenol derivatives.
Figures
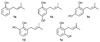

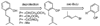
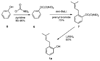
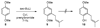
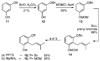
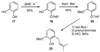
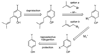
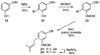
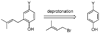
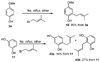
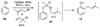
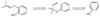
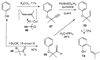
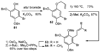
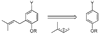
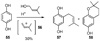
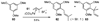
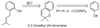
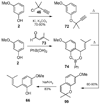
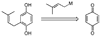

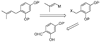
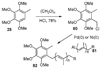
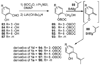
References
-
- Brandt U. Biofactors. 1999;9:95. - PubMed
-
- Fukui H, Feroj Hassan AFM, Ueoka T, Kyo M. Phytochemistry. 1998;47:1037.
-
- Mori K, Waku M, Sakakibara M. Tetrahedron. 1985;41:2825.
-
- Basset C, Sherwood RT, Kepler JA, Hamilton PB. Phytopathology. 1967;57:1046.
- Wächter GA, Hoffmann JJ, Furbacher T, Blake ME, Timmermann BN. Phytochemistry. 1999;52:1469. - PubMed
-
- Ghirtis K, Pouli N, Marakos P, Skaltsounis A-L, Leonce S, Caignard DH, Atassi G. Heterocycles. 2000;53:93. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
