Regulation of the meiotic prophase I to metaphase I transition in mouse spermatocytes
- PMID: 18563426
- PMCID: PMC2737826
- DOI: 10.1007/s00412-008-0167-3
Regulation of the meiotic prophase I to metaphase I transition in mouse spermatocytes
Abstract
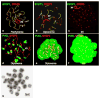
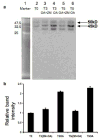
The meiotic prophase I to metaphase I transition (G2/MI) involves disassembly of synaptonemal complex (SC), chromatin condensation, and final compaction of morphologically distinct MI bivalent chromosomes. Control of these processes is poorly understood. The G2/MI transition was experimentally induced in mouse pachytene spermatocytes by okadaic acid (OA), and kinetic analysis revealed that disassembly of the central element of the SC occurred very rapidly after OA treatment, before histone H3 phosphorylation on Ser10. These events were followed by relocalization of SYCP3 and final condensation of bivalents. Enzymatic control of these G2/MI transition events was studied using small molecule inhibitors: butyrolactone I (BLI), an inhibitor of cyclin-dependent kinases (CDKs) and ZM447439 (ZM), an inhibitor of aurora kinases (AURKs). The formation of highly condensed MI bivalents and disassembly of the SC are regulated by both CDKs and AURKs. AURKs also mediate phosphorylation of histone H3 in meiosis. However, neither BLI nor ZM inhibited disassembly of the central element of the SC. Thus, despite evidence that the metaphase promoting factor is a universal regulator of the onset of cell division, desynapsis, the first and key step of the G2/MI transition, occurs independently of BLI-sensitive CDKs and ZM-sensitive AURKs.
Figures






References
-
- Allen JW, Dix DJ, Collins BW, Merrick BA, He C, Selkirk JK, Poorman-Allen P, Dresser ME, Eddy EM. Hsp70-2 is part of the synaptonemal complex in mouse and hamster spermatocytes. Chromosoma. 1996;104:414–421. - PubMed
-
- Arlot-Bonnemains Y, Klotzbucher A, Giet R, Uzbekov R, Bihan R, Prigent C. Identification of a functional destruction box in the Xenopus laevis aurora -A kinase pEg2. FEBS Lett. 2001;508:149–152. - PubMed
-
- Ashley T, Gaeth AP, Creemers LB, Hack AM, De Rooij DG. Correlation of meiotic events in testis sections and microspreads of mouse spermatocytes relative to the mid-pachytene checkpoint. Chromosoma. 2004;113:126–136. - PubMed
-
- Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis. 2004;40:184–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

