Diverse synaptic terminals on rat stapedius motoneurons
- PMID: 18563488
- PMCID: PMC2538147
- DOI: 10.1007/s10162-008-0125-z
Diverse synaptic terminals on rat stapedius motoneurons
Abstract
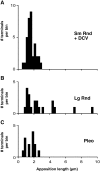
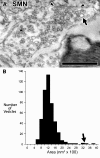
Stapedius motoneurons (SMN) mediate the contraction of the stapedius muscle, which protects the inner ear from injury and reduces the masking effects of background noise. A variety of inputs to SMNs are known to exist, but their terminal ultrastructure has not been investigated. We characterized the synaptic terminals on retrogradely labeled SMNs found just ventromedial to the facial motor nucleus. About 80% of the terminals contained round synaptic vesicles. One type (Sm Rnd) had small, round vesicles filling the terminal with occasional dense core vesicles and formed an asymmetric synapse. Sm Rnd terminals were small with lengths of apposition to the SMN less than 3 microm. Partial reconstructions from serial sections demonstrated that these terminals formed up to three synapses per terminal. Another terminal type (Lg Rnd) had large, round vesicles and asymmetric synapses. Most Lg Rnd terminals were small but some were extensive, e.g., abutting the SMN for up to 10 microm. One of these terminals formed at least seven synapses. Another terminal type (Pleo) had pleomorphic vesicles and symmetric active zones that, in some cases, were invaginated by spines from the SMN. A fourth uncommon terminal type (Het Rnd) had round vesicles of heterogeneous sizes and asymmetric synapses. A fifth rare terminal type (Cist) had large, round vesicles and an accompanying subsurface cistern in the SMN. These were generally the same kinds of terminals found on other motoneurons, but the high proportion of round vesicle synapses indicate that SMNs receive mostly excitatory inputs.
Figures






Similar articles
-
Tensor tympani motoneurons receive mostly excitatory synaptic inputs.Anat Rec (Hoboken). 2013 Jan;296(1):133-45. doi: 10.1002/ar.22620. Epub 2012 Nov 15. Anat Rec (Hoboken). 2013. PMID: 23165747 Free PMC article.
-
Auditory brainstem circuits that mediate the middle ear muscle reflex.Trends Amplif. 2010 Sep;14(3):170-91. doi: 10.1177/1084713810381771. Epub 2010 Sep 23. Trends Amplif. 2010. PMID: 20870664 Free PMC article. Review.
-
Direct synaptic projections to esophageal motoneurons in the nucleus ambiguus from the nucleus of the solitary tract of the rat.J Comp Neurol. 1997 Apr 28;381(1):18-30. doi: 10.1002/(sici)1096-9861(19970428)381:1<18::aid-cne2>3.0.co;2-n. J Comp Neurol. 1997. PMID: 9087416
-
Synaptology of the direct projections from the nucleus of the solitary tract to pharyngeal motoneurons in the nucleus ambiguus of the rat.J Comp Neurol. 1998 Apr 13;393(3):391-401. J Comp Neurol. 1998. PMID: 9548557
-
Ultrastructural aspects of the coeruleo-spinal projection.Prog Brain Res. 1991;88:143-56. doi: 10.1016/s0079-6123(08)63804-2. Prog Brain Res. 1991. PMID: 1687617 Review.
Cited by
-
Benefits of Stimulus Exposure: Developmental Learning Independent of Task Performance.Front Neurosci. 2016 Jun 17;10:263. doi: 10.3389/fnins.2016.00263. eCollection 2016. Front Neurosci. 2016. PMID: 27378837 Free PMC article.
-
Ultrastructure of spines and associated terminals on brainstem neurons controlling auditory input.Brain Res. 2013 Jun 21;1516:1-10. doi: 10.1016/j.brainres.2013.04.020. Epub 2013 Apr 18. Brain Res. 2013. PMID: 23602963 Free PMC article.
-
Tensor tympani motoneurons receive mostly excitatory synaptic inputs.Anat Rec (Hoboken). 2013 Jan;296(1):133-45. doi: 10.1002/ar.22620. Epub 2012 Nov 15. Anat Rec (Hoboken). 2013. PMID: 23165747 Free PMC article.
-
Auditory brainstem circuits that mediate the middle ear muscle reflex.Trends Amplif. 2010 Sep;14(3):170-91. doi: 10.1177/1084713810381771. Epub 2010 Sep 23. Trends Amplif. 2010. PMID: 20870664 Free PMC article. Review.
-
Ultrastructure of cisternal synapses on outer hair cells of the mouse cochlea.J Comp Neurol. 2014 Feb 15;522(3):717-29. doi: 10.1002/cne.23478. J Comp Neurol. 2014. PMID: 24122766 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/cne.21118', 'is_inner': False, 'url': 'https://doi.org/10.1002/cne.21118'}, {'type': 'PubMed', 'value': '16977616', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16977616/'}]}
- Benson TE, Brown MC. Ultrastructure of synaptic input to medial olivocochlear neurons. J. Comp. Neurol 499:244–257, 2006. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/(SICI)1096-9861(19960129)365:1<27::AID-CNE3>3.0.CO;2-L', 'is_inner': False, 'url': 'https://doi.org/10.1002/(sici)1096-9861(19960129)365:1<27::aid-cne3>3.0.co;2-l'}, {'type': 'PubMed', 'value': '8821439', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8821439/'}]}
- Benson TE, Berglund AM, Brown MC. Synaptic input to cochlear nucleus dendrites that receive medial olivocochlear synapses. J. Comp. Neurol 365:27–41, 1996. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/cne.903590208', 'is_inner': False, 'url': 'https://doi.org/10.1002/cne.903590208'}, {'type': 'PubMed', 'value': '7499530', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7499530/'}]}
- Blok BF, De Weerd H, Holstege G. Ultrastructural evidence for a paucity of projections from the lumbosacral cord to the pontine micturition center or M-region in the cat: A new concept for the organization of the micturition reflex with the periaqueductal gray as central relay. J. Comp. Neurol 359:300–309, 1995. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/0006-8993(73)90404-6', 'is_inner': False, 'url': 'https://doi.org/10.1016/0006-8993(73)90404-6'}, {'type': 'PubMed', 'value': '4349006', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/4349006/'}]}
- Borg E. On the neuronal organization of the acoustic middle ear reflex: A physiological and anatomical study. Brain Res 49:101–123, 1973. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.3109/00016487409126341', 'is_inner': False, 'url': 'https://doi.org/10.3109/00016487409126341'}, {'type': 'PubMed', 'value': '4432740', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/4432740/'}]}
- Borg E, Zakrisson JE. Stapedius reflex and monaural masking. Acta Otolaryngol 78:155–161, 1974. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

