Niosome-encapsulated gentamicin for ophthalmic controlled delivery
- PMID: 18563578
- PMCID: PMC2977028
- DOI: 10.1208/s12249-008-9105-1
Niosome-encapsulated gentamicin for ophthalmic controlled delivery
Abstract
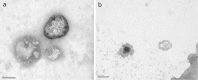
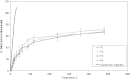
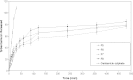
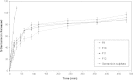
The objective of the present research was to investigate the feasibility of using non-ionic surfactant vesicles (niosomes) as carriers for the ophthalmic controlled delivery of a water soluble local antibiotic; gentamicin sulphate. Niosomal formulations were prepared using various surfactants (Tween 60, Tween 80 or Brij 35), in the presence of cholesterol and a negative charge inducer dicetyl phosphate (DCP) in different molar ratios and by employing a thin film hydration technique. The ability of these vesicles to entrap the studied drug was evaluated by determining the entrapment efficiency %EE after centrifugation and separation of the formed vesicles. Photomicroscopy and transmission electron microscopy as well as particle size analysis were used to study the formation, morphology and size of the drug loaded niosomes. Results showed a substantial change in the release rate and an alteration in the %EE of gentamicin sulphate from niosomal formulations upon varying type of surfactant, cholesterol content and presence or absence of DCP. In-vitro drug release results confirmed that niosomal formulations have exhibited a high retention of gentamicin sulphate inside the vesicles such that their in vitro release was slower compared to the drug solution. A preparation with 1:1:0.1 molar ratio of Tween 60, cholesterol and DCP gave the most advantageous entrapment (92.02% +/- 1.43) and release results (Q(8h) = 66.29% +/- 1.33) as compared to other compositions. Ocular irritancy test performed on albino rabbits, showed no sign of irritation for all tested niosomal formulations.
Figures

Similar articles
-
Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: intraocular pressure measurement in white albino rabbits.Drug Deliv. 2020 Dec;27(1):888-899. doi: 10.1080/10717544.2020.1775726. Drug Deliv. 2020. PMID: 32551978 Free PMC article.
-
Effect of formulation compositions on niosomal preparations.Pharm Dev Technol. 2013 May-Jun;18(3):667-72. doi: 10.3109/10837450.2012.672988. Epub 2012 Apr 2. Pharm Dev Technol. 2013. PMID: 22468904
-
Formulation and evaluation of metformin hydrochloride-loaded niosomes as controlled release drug delivery system.Drug Deliv. 2013 Apr-May;20(3-4):120-6. doi: 10.3109/10717544.2013.779332. Epub 2013 May 8. Drug Deliv. 2013. PMID: 23651102
-
Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications.Eur J Pharm Biopharm. 2019 Nov;144:18-39. doi: 10.1016/j.ejpb.2019.08.015. Epub 2019 Aug 22. Eur J Pharm Biopharm. 2019. PMID: 31446046 Review.
-
Quality by design for Niosome-Based nanocarriers to improve transdermal drug delivery from lab to industry.Int J Pharm. 2024 Dec 5;666:124747. doi: 10.1016/j.ijpharm.2024.124747. Epub 2024 Sep 24. Int J Pharm. 2024. PMID: 39326474 Review.
Cited by
-
Span 60/Cholesterol Niosomal Formulation as a Suitable Vehicle for Gallic Acid Delivery with Potent In Vitro Antibacterial, Antimelanoma, and Anti-Tyrosinase Activity.Pharmaceuticals (Basel). 2023 Dec 2;16(12):1680. doi: 10.3390/ph16121680. Pharmaceuticals (Basel). 2023. PMID: 38139807 Free PMC article.
-
Eye drop delivery of pigment epithelium-derived factor-34 promotes retinal ganglion cell neuroprotection and axon regeneration.Mol Cell Neurosci. 2015 Sep;68:212-21. doi: 10.1016/j.mcn.2015.08.001. Epub 2015 Aug 8. Mol Cell Neurosci. 2015. PMID: 26260110 Free PMC article.
-
Potentiality of Melittin-Loaded Niosomal Vesicles Against Vancomycin-Intermediate Staphylococcus aureus and Staphylococcal Skin Infection.Int J Nanomedicine. 2021 Nov 16;16:7639-7661. doi: 10.2147/IJN.S325901. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34819727 Free PMC article.
-
Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases.Pharmaceutics. 2018 Feb 27;10(1):28. doi: 10.3390/pharmaceutics10010028. Pharmaceutics. 2018. PMID: 29495528 Free PMC article. Review.
-
Ophthalmic Drug Delivery Systems for Antibiotherapy-A Review.Pharmaceutics. 2018 Jan 13;10(1):10. doi: 10.3390/pharmaceutics10010010. Pharmaceutics. 2018. PMID: 29342879 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

