Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease
- PMID: 18563844
- PMCID: PMC3753011
- DOI: 10.1002/hep.22390
Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease
Abstract
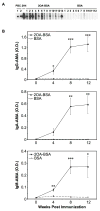
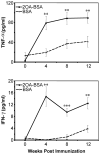
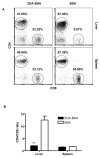
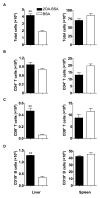
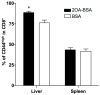
There have been important advances in defining effector mechanisms for several human autoimmune diseases. However, for most human autoimmune diseases, the induction stage is less well defined and there are very few clues on etiology. Our laboratory has focused on defining the molecular basis of autoantibody recognition and epitope modification in primary biliary cirrhosis (PBC). Our work has demonstrated that antibodies to mitochondria, the hallmark of disease, are directed against a very conserved site of pyruvate dehydrogenase, the E2 subunit of pyruvate dehydrogenase (PDC-E2). We have also demonstrated that several chemical xenobiotics, chosen based on quantitative structural activity relationship analysis and rigorous epitope analysis, when coupled to the lysine residue that normally binds the lipoic acid cofactor of PDC-E2, reacts as well or better to PBC sera than native autoantigen. In the present studies, we immunized C57BL/6 mice with one such xenobiotic, 2-octynoic acid, coupled to bovine serum albumin and we followed the mice for 24 weeks. Animals were studied for appearance of histologic lesions as well as appearance of antibodies to PDC-E2, serum levels of tumor necrosis factor-alpha and interferon-gamma, and splenic and liver lymphoid phenotyping by flow cytometry. Mice immunized with 2-octynoic acid manifest autoimmune cholangitis, typical mitochondrial autoantibodies, increased liver lymphoid cell numbers, an increase in CD8(+) liver infiltrating cells, particularly CD8(+) T cells that coexpress CD44, and finally an elevation of serum tumor necrosis factor-alpha and interferon-gamma.
Conclusion: these data provide a persuasive argument in favor of an environmental origin for human PBC.
Conflict of interest statement
No conflicts of interest exist
Figures

Comment in
-
The specificity of liver inflammation in mouse models of primary biliary cirrhosis.Hepatology. 2008 Oct;48(4):1353-4; author reply 1354-5. doi: 10.1002/hep.22522. Hepatology. 2008. PMID: 18792128 No abstract available.
Similar articles
-
The multi-hit hypothesis of primary biliary cirrhosis: polyinosinic-polycytidylic acid (poly I:C) and murine autoimmune cholangitis.Clin Exp Immunol. 2011 Oct;166(1):110-20. doi: 10.1111/j.1365-2249.2011.04453.x. Clin Exp Immunol. 2011. PMID: 21910728 Free PMC article.
-
Electrophile-modified lipoic derivatives of PDC-E2 elicits anti-mitochondrial antibody reactivity.J Autoimmun. 2011 Nov;37(3):209-16. doi: 10.1016/j.jaut.2011.06.001. Epub 2011 Jul 18. J Autoimmun. 2011. PMID: 21763105 Free PMC article.
-
Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid.J Immunol. 2005 May 1;174(9):5874-83. doi: 10.4049/jimmunol.174.9.5874. J Immunol. 2005. PMID: 15845458
-
Xenobiotics and loss of tolerance in primary biliary cholangitis.World J Gastroenterol. 2016 Jan 7;22(1):338-48. doi: 10.3748/wjg.v22.i1.338. World J Gastroenterol. 2016. PMID: 26755880 Free PMC article. Review.
-
Autoreactive responses to pyruvate dehydrogenase complex in the pathogenesis of primary biliary cirrhosis.Immunol Rev. 2000 Apr;174:238-49. doi: 10.1034/j.1600-0528.2002.00021h.x. Immunol Rev. 2000. PMID: 10807520 Review.
Cited by
-
Role of autoimmunity in primary biliary cirrhosis.World J Gastroenterol. 2012 Dec 28;18(48):7141-8. doi: 10.3748/wjg.v18.i48.7141. World J Gastroenterol. 2012. PMID: 23326118 Free PMC article. Review.
-
Peak inflammation in atherosclerosis, primary biliary cirrhosis and autoimmune arthritis is counter-intuitively associated with regulatory T cell enrichment.Immunobiology. 2015 Aug;220(8):1025-9. doi: 10.1016/j.imbio.2015.02.006. Epub 2015 Feb 26. Immunobiology. 2015. PMID: 25770018 Free PMC article.
-
Infection as a cause of type 1 diabetes?Curr Opin Rheumatol. 2012 Jul;24(4):417-23. doi: 10.1097/BOR.0b013e3283533719. Curr Opin Rheumatol. 2012. PMID: 22504578 Free PMC article. Review.
-
Immunological potential of cytotoxic T lymphocyte antigen 4 immunoglobulin in murine autoimmune cholangitis.Clin Exp Immunol. 2015 Jun;180(3):371-82. doi: 10.1111/cei.12581. Clin Exp Immunol. 2015. PMID: 25581259 Free PMC article.
-
Environmental risk factors and comorbidities of primary biliary cholangitis in Korea: a case-control study.Korean J Intern Med. 2021 Mar;36(2):313-321. doi: 10.3904/kjim.2019.234. Epub 2020 Mar 26. Korean J Intern Med. 2021. PMID: 32204000 Free PMC article.
References
-
- Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH, Kurth MJ, Gershwin ME. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–7. - PubMed
-
- Padgett KA, Selmi C, Kenny TP, Leung PS, Balkwill DL, Ansari AA, Coppel RL, Gershwin ME. Phylogenetic and immunological definition of four lipoylated proteins from Novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J Autoimmun. 2005;24:209–19. - PubMed
-
- Long SA, Quan C, Van de Water J, Nantz MH, Kurth MJ, Barsky D, Colvin ME, Lam KS, Coppel RL, Ansari A, Gershwin ME. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167:2956–63. - PubMed
-
- Leung PS, Quan C, Park O, Van de Water J, Kurth MJ, Nantz MH, Ansari AA, Coppel RL, Lam KS, Gershwin ME. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170:5326–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
