In situ cleavage of the acidic domain from the p115 tether inhibits exocytic transport
- PMID: 18564369
- PMCID: PMC3035718
- DOI: 10.1111/j.1600-0854.2008.00783.x
In situ cleavage of the acidic domain from the p115 tether inhibits exocytic transport
Abstract
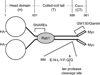
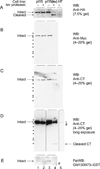
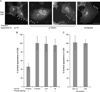
Golgins are coiled-coil proteins involved in Golgi architecture and function. A complex of golgins (p115, GM130 and giantin), together with the rab1 guanosine triphosphatase and cis Golgi SNAREs, helps to mediate fusion processes at the entry face of the Golgi apparatus. The C-terminal acidic domain of p115 binds specifically to GM130 and giantin. However, deletion of this domain in vivo appears to have no effect on exocytic transport when using an RNA interference depletion/rescue approach (Puthenveedu MA, Linstedt AD. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc Natl Acad Sci U S A 2004;101:1253-1256). In this study, we have used a different approach introducing a tobacco etch virus (tev) protease cleavage site into p115 so that the C-terminal domain can be rapidly and specifically released in vivo by microinjection of the tev protease. The results show that cleavage inhibits exocytic transport to the cell surface.
Figures

Similar articles
-
A cryptic Rab1-binding site in the p115 tethering protein.J Biol Chem. 2005 Jul 8;280(27):25840-8. doi: 10.1074/jbc.M503925200. Epub 2005 May 6. J Biol Chem. 2005. PMID: 15878873
-
Binding relationships of membrane tethering components. The giantin N terminus and the GM130 N terminus compete for binding to the p115 C terminus.J Biol Chem. 2000 Apr 7;275(14):10196-201. doi: 10.1074/jbc.275.14.10196. J Biol Chem. 2000. PMID: 10744704
-
The p115-interactive proteins GM130 and giantin participate in endoplasmic reticulum-Golgi traffic.J Biol Chem. 2001 Jan 26;276(4):2693-700. doi: 10.1074/jbc.M007957200. Epub 2000 Oct 16. J Biol Chem. 2001. PMID: 11035033
-
Golgins in the structure and dynamics of the Golgi apparatus.Curr Opin Cell Biol. 2003 Aug;15(4):405-13. doi: 10.1016/s0955-0674(03)00054-1. Curr Opin Cell Biol. 2003. PMID: 12892780 Review.
-
Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions.J Pharmacol Sci. 2010;112(3):255-64. doi: 10.1254/jphs.09r03cr. Epub 2010 Mar 2. J Pharmacol Sci. 2010. PMID: 20197635 Review.
Cited by
-
The golgin coiled-coil proteins of the Golgi apparatus.Cold Spring Harb Perspect Biol. 2011 Jun 1;3(6):a005256. doi: 10.1101/cshperspect.a005256. Cold Spring Harb Perspect Biol. 2011. PMID: 21436057 Free PMC article. Review.
-
Structural and functional analysis of the globular head domain of p115 provides insight into membrane tethering.J Mol Biol. 2009 Aug 7;391(1):26-41. doi: 10.1016/j.jmb.2009.04.062. Epub 2009 May 4. J Mol Biol. 2009. PMID: 19414022 Free PMC article.
-
p115-SNARE interactions: a dynamic cycle of p115 binding monomeric SNARE motifs and releasing assembled bundles.Traffic. 2015 Feb;16(2):148-71. doi: 10.1111/tra.12242. Epub 2015 Jan 4. Traffic. 2015. PMID: 25406594 Free PMC article.
-
Unusual armadillo fold in the human general vesicular transport factor p115.PLoS One. 2009;4(2):e4656. doi: 10.1371/journal.pone.0004656. Epub 2009 Feb 27. PLoS One. 2009. PMID: 19247479 Free PMC article.
-
Armadillo motifs involved in vesicular transport.PLoS One. 2010 Feb 1;5(2):e8991. doi: 10.1371/journal.pone.0008991. PLoS One. 2010. PMID: 20126549 Free PMC article.
References
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116(2):153–166. - PubMed
-
- Barlowe C. Traffic COPs of the early secretory pathway. Traffic. 2000;1(5):371–377. - PubMed
-
- Watson P, Stephens DJ. ER-to-Golgi transport: form and formation of vesicular and tubular carriers. Biochim Biophys Acta. 2005;1744(3):304–315. - PubMed
-
- Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. - PubMed
-
- Pelham HR, Rothman JE. The debate about transport in the Golgi--two sides of the same coin? Cell. 2000;102(6):713–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

