CD1a and MHC class I follow a similar endocytic recycling pathway
- PMID: 18564371
- PMCID: PMC3839101
- DOI: 10.1111/j.1600-0854.2008.00781.x
CD1a and MHC class I follow a similar endocytic recycling pathway
Abstract
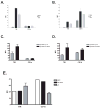
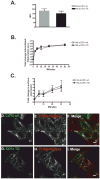
CD1 proteins are a family of major histocompatibility complex (MHC) class I-like antigen-presenting molecules that present lipids to T cells. The cytoplasmic tails (CTs) of all human CD1 isoforms, with the exception of CD1a, contain tyrosine-based sorting motifs, responsible for the internalization of proteins by the clathrin-mediated pathway. The role of the CD1a CT, which does not possess any sorting motifs, as well as its mode of internalization are not known. We investigated the internalization and recycling pathways followed by CD1a and the role of its CT. We found that CD1a can be internalized by a clathrin- and dynamin-independent pathway and that it follows a Rab22a- and ADP ribosylation factor (ARF)6-dependent recycling pathway, similar to other cargo internalized independent of clathrin. We also found that the CD1a CT is S-acylated. However, this posttranslational modification does not determine the rate of internalization or recycling of the protein or its localization to detergent-resistant membrane microdomains (DRMs) where we found CD1a to be enriched. We also show that plasma membrane DRMs are essential for efficient CD1a-mediated antigen presentation. These findings place CD1a closer to MHC class I in its trafficking and potential antigen-loading compartments among CD1 isoforms. Furthermore, we identify CD1a as a new marker for the clathrin- and dynamin-independent and DRM-dependent pathway of internalization as well as the Rab22a- and ARF6-dependent recycling pathway.
Figures


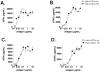



References
-
- Dascher CC. Evolutionary biology of CD1. Curr Top Microbiol Immunol. 2007;314:3–26. - PubMed
-
- Zajonc DM, Wilson IA. Architecture of CD1 proteins. Curr Top Microbiol Immunol. 2007;314:27–50. - PubMed
-
- Young DC, Moody DB. T-cell recognition of glycolipids presented by CD1 proteins. Glycobiology. 2006;16(7):103R–112R. - PubMed
-
- Moody DB, Briken V, Cheng TY, Roura-Mir C, Guy MR, Geho DH, Tykocinski ML, Besra GS, Porcelli SA. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat Immunol. 2002;3(5):435–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

