The MACPF/CDC family of pore-forming toxins
- PMID: 18564372
- PMCID: PMC2654483
- DOI: 10.1111/j.1462-5822.2008.01191.x
The MACPF/CDC family of pore-forming toxins
Abstract
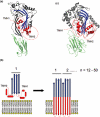
Pore-forming toxins (PFTs) are commonly associated with bacterial pathogenesis. In eukaryotes, however, PFTs operate in the immune system or are deployed for attacking prey (e.g. venoms). This review focuses upon two families of globular protein PFTs: the cholesterol-dependent cytolysins (CDCs) and the membrane attack complex/perforin superfamily (MACPF). CDCs are produced by Gram-positive bacteria and lyse or permeabilize host cells or intracellular organelles during infection. In eukaryotes, MACPF proteins have both lytic and non-lytic roles and function in immunity, invasion and development. The structure and molecular mechanism of several CDCs are relatively well characterized. Pore formation involves oligomerization and assembly of soluble monomers into a ring-shaped pre-pore which undergoes conformational change to insert into membranes, forming a large amphipathic transmembrane beta-barrel. In contrast, the structure and mechanism of MACPF proteins has remained obscure. Recent crystallographic studies now reveal that although MACPF and CDCs are extremely divergent at the sequence level, they share a common fold. Together with biochemical studies, these structural data suggest that lytic MACPF proteins use a CDC-like mechanism of membrane disruption, and will help understand the roles these proteins play in immunity and development.
Figures
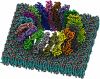
References
-
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–972. - PubMed
-
- Alouf JE, Popoff MR. The Comprehensive Sourcebook of Bacterial Protein Toxins Amsterdam. Boston, MA: Elsevier; 2006. p. xxiii.
-
- Aroian R, van der Goot FG. Pore-forming toxins and cellular non-immune defenses (CNIDs) Curr Opin Microbiol. 2007;10:57–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

