Barriers for retinal gene therapy: separating fact from fiction
- PMID: 18565565
- PMCID: PMC2538423
- DOI: 10.1016/j.visres.2008.05.005
Barriers for retinal gene therapy: separating fact from fiction
Abstract
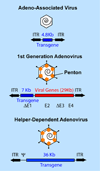
The majority of recent preclinical gene therapy studies targeting the retina have used adeno-associated virus (AAV) as the gene transfer vector. However, AAV has several limitations including the ability to generate innate inflammatory responses, the ability to cause insertional mutagenesis at a frequency of up to 56% in some tissues and a limited cloning capacity of 4.8Kb. Furthermore, AAV is known to generate limiting immune responses in humans despite the absence of similar immune responses in preclinical canine and murine studies. Three clinical trials to treat Leber's congenital amaurosis using AAV are under way. A clinical trial to treat Stargardt's using lentivirus vectors has also been recently announced. However, very limited evidence currently exists that lentivirus vectors can efficiently transduce photoreceptor cells. In contrast, very few preclinical ocular gene therapy studies have utilized adenovirus as the gene therapy vector. Nonetheless, the only two ocular gene therapy clinical trials performed to date have each used adenovirus as the vector and more significantly, in these published trials there has been no observed serious adverse event. These trials appear to be poised for Phase II/III status. Activation of cytotoxic T lymphocytes limits duration of transgene expression in the retina from first generation adenovirus vectors. However, an advanced class of adenovirus vectors referred to as Helper-dependent Adenovirus (Hd-Ad) have recently been shown to be capable of expressing transgenes in ocular tissues for more than one year. Hd-Ad vectors have many properties that potentially warrant their inclusion in the retinal gene therapy toolbox for the treatment of retinal degenerative diseases.
Figures
Similar articles
-
[Developments in gene delivery vectors for ocular gene therapy].Med Sci (Paris). 2015 May;31(5):529-37. doi: 10.1051/medsci/20153105015. Epub 2015 Jun 9. Med Sci (Paris). 2015. PMID: 26059304 Review. French.
-
Screening of a retinal-targeting Adeno-Associated Virus (AAV) via DNA shuffling.Exp Eye Res. 2025 Feb;251:110245. doi: 10.1016/j.exer.2025.110245. Epub 2025 Jan 21. Exp Eye Res. 2025. PMID: 39848559
-
Molecular Engineering of Adeno-Associated Virus Capsid Improves Its Therapeutic Gene Transfer in Murine Models of Hemophilia and Retinal Degeneration.Mol Pharm. 2019 Nov 4;16(11):4738-4750. doi: 10.1021/acs.molpharmaceut.9b00959. Epub 2019 Oct 22. Mol Pharm. 2019. PMID: 31596095 Free PMC article.
-
Highly efficient retinal gene delivery with helper-dependent adenoviral vectors.Genes Dis. 2014 Dec;1(2):227-237. doi: 10.1016/j.gendis.2014.09.002. Genes Dis. 2014. PMID: 26161435 Free PMC article.
-
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression.Int J Nanomedicine. 2024 Jul 29;19:7691-7708. doi: 10.2147/IJN.S459905. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099791 Free PMC article. Review.
Cited by
-
Gene replacement therapy for retinal CNG channelopathies.Mol Genet Genomics. 2013 Oct;288(10):459-67. doi: 10.1007/s00438-013-0766-4. Epub 2013 Jul 17. Mol Genet Genomics. 2013. PMID: 23861024 Review.
-
Helper-Dependent Adenoviral Vectors.J Genet Syndr Gene Ther. 2011 Oct 29;Suppl 5:001. doi: 10.4172/2157-7412.s5-001. J Genet Syndr Gene Ther. 2011. PMID: 24533227 Free PMC article.
-
Peripheral monocytes and neutrophils promote photoreceptor cell death in an experimental retinal detachment model.Cell Death Dis. 2023 Dec 16;14(12):834. doi: 10.1038/s41419-023-06350-6. Cell Death Dis. 2023. PMID: 38102109 Free PMC article.
-
RIPK necrotic cell death pathway in both donor photoreceptor and host immune cells synergize to affect photoreceptor graft survival.FASEB J. 2023 Apr;37(4):e22847. doi: 10.1096/fj.202201137R. FASEB J. 2023. PMID: 36862516 Free PMC article.
-
Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms.Front Mol Neurosci. 2015 May 18;8:14. doi: 10.3389/fnmol.2015.00014. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26041987 Free PMC article. Review.
References
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. - PubMed
-
- Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K, Karpati G. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. HumMolGenet. 1994;3:579–584. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Ali RR, Reichel MB, Thrasher AJ, Levinsky RJ, Kinnon C, Kanuga N, Hunt DM, Bhattacharya SS. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum Mol Genet. 1996;5:591–594. - PubMed
-
- Ali RR, Sarra GM, Stephens C, Alwis MD, Bainbridge JW, Munro PM, Fauser S, Reichel MB, Kinnon C, Hunt DM, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. [see comments] - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

