Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta
- PMID: 18565769
- PMCID: PMC2605974
- DOI: 10.1016/j.joca.2008.04.027
Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta
Abstract
Objective: To provide a more complete picture of the effect of interleukin-1 beta (IL-1beta) on adult human articular chondrocyte gene expression, in contrast to the candidate gene approach.
Design: Chondrocytes from human knee cartilage were cultured in medium containing IL-1beta. Changes in gene expression were analyzed by microarray and reverse transcriptase-polymerase chain reaction analysis. The ability of transforming growth factor beta-1 (TGF-beta1), fibroblast growth factor (FGF)-18, and bone morphogenetic protein 2 (BMP-2) to alter the effects of IL-1beta was analyzed. Computational analysis of the promoter regions of differentially expressed genes for transcription factor binding motifs was performed.
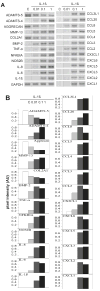
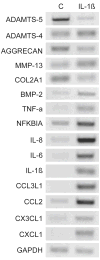
Results: IL-1beta-treated human chondrocytes showed significant increases in the expression of granulocyte colony stimulating factor-3, endothelial leukocyte adhesion molecule 1 and leukemia inhibitory factor as well as for a large group of chemokines that include CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8, CCL2, CCL3, CCL4, CCL5, CCL8, CCL20, CCL3L1, CX3CL1 and the cytokine IL-6. As expected, the mRNA for matrix metalloproteinase (MMP)-13 and BMP-2 also increased while mRNA for the matrix genes COL2A1 and aggrecan was down-regulated. A subset of chemokines increased rapidly at very low levels of IL-1beta. The phenotype induced by IL-1beta was partially reversed by TGF-beta1, but not by BMP-2. In the presence of IL-1beta, FGF-18 increased expression of ADAMTS-4, aggrecan, BMP-2, COL2A1, CCL3, CCL4, CCL20, CXCL1, CXCL3, CXCL6, IL-1beta, IL-6, and IL-8 and decreased ADAMTS-5, MMP-13, CCL2, and CCL8. Computational analysis revealed a high likelihood that the most up-regulated chemokines are regulated by the transcription factors myocyte enhancer binding factor-3 (MEF-3), CCAAT/enhancer binding protein (C/EBP) and nuclear factor-kappa B (NF-kappaB).
Conclusion: IL-1beta has a diverse effect on gene expression profile in human chondrocytes affecting matrix genes as well as chemokines and cytokines. TGF-beta1 has the ability to antagonize some of the phenotype induced by IL-1beta.
Figures





References
-
- Poole AR, Guilak F, Abramson S. Etiopathogenesis of osteoarthritis. In: Moskowitz RW, Altman RD, Hochberg MC, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: Diagnosis and Medical/Surgical Management. 4. Philadelphia: Kluwer; 2007. pp. 27–49.
-
- Sandell LJ, Heinegard D, Hering TM. Cell Biology, Biochemistry, and Molecular Biology of Articular Cartilage in Osteoarthritis. In: Moskowitz RW, Altman RD, Hochberg MC, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: Diagnosis and Medical/Surgical Management. 4. Philadelphia: Kluwer; 2007. pp. 73–106.
-
- Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am. 2003;85-A(Suppl 3):59–66. - PubMed
-
- Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Frontiers in Bioscience. 1999;4:694–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

