A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome
- PMID: 18565853
- PMCID: PMC4081352
- DOI: 10.1182/blood-2007-11-124602
A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome
Abstract
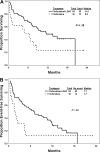
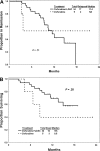
We previously reported the feasibility of clofarabine and cytarabine combinations in AML. Questions remain as to (1) the therapeutic advantage of this combination and (2) the role of lower doses of clofarabine and cytarabine in older patients. We have conducted an adaptively randomized study of lower-dose clofarabine with or without low-dose cytarabine in previously untreated patients with AML aged 60 years and older. Patients received 30 mg/m(2) clofarabine intravenously daily for 5 days with or without 20 mg/m(2) cytarabine subcutaneously daily for 14 days as induction. Consolidation consisted of 3 days of clofarabine with or without 7 days of cytarabine. Seventy patients were enrolled. The median age was 71 years (range, 60-83 years). Sixteen patients received clofarabine and 54 the combination. Overall, 56% achieved complete remission (CR). CR rate was significantly higher with the combination (63% vs 31%; P = .025). Induction mortality was 19% with the combination versus 31% with clofarabine alone (P = .276). The combination showed better event-free survival (7.1 months vs 1.7 months; P = .04), but not overall survival (11.4 months vs 5.8 months; P = .1). Clofarabine plus low-dose cytarabine has a higher response rate than clofarabine alone with comparable toxicity. This trial is registered at www.clinicaltrials.gov as no. NCT00088218.
Figures
References
-
- Estey EH. General approach to, and perspectives on clinical research in, older patients with newly diagnosed acute myeloid leukemia. Semin Hematol. 2006;43:89–95. - PubMed
-
- Burnett AK, Mohite U. Treatment of older patients with acute myeloid leukemia—new agents. Semin Hematol. 2006;43:96–106. - PubMed
-
- Montgomery JA, Shortnacy-Fowler AT, Clayton SD, et al. Synthesis and biologic activity of 2′-fluoro-2-halo derivatives of 9-β-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. - PubMed
-
- Parker WB, Allan PW, Hassan AE, et al. Antitumor activity of 2-fluoro-2′-deoxyadenosine against tumors that express Escherichia coli purine nucleoside phosphorylase. Cancer Gene Ther. 2003;10:23–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous