Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma
- PMID: 18565886
- PMCID: PMC3635806
- DOI: 10.1200/JCO.2007.15.9947
Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma
Abstract
Purpose: To determine the clinical and biologic effects of bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, in unresectable hepatocellular carcinoma (HCC).
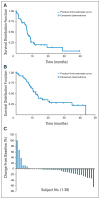
Patients and methods: Adults with organ-confined HCC, Eastern Cooperative Oncology Group performance status of 0 to 2, and compensated liver disease were eligible. Patients received bevacizumab 5 mg/kg (n = 12) or 10 mg/kg (n = 34) every 2 weeks until disease progression or treatment-limiting toxicity. The primary objective was to determine whether bevacizumab improved the 6-month progression-free survival (PFS) rate from 40% to 60%. Secondary end points included determining the effects of bevacizumab on arterial enhancement and on plasma cytokine levels and the capacity of patients' plasma to support angiogenesis via an in vitro assay.
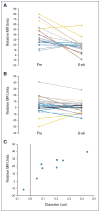
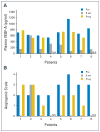
Results: The study included 46 patients, of whom six had objective responses (13%; 95% CI, 3% to 23%), and 65% were progression free at 6 months. Median PFS time was 6.9 months (95% CI, 6.5 to 9.1 months); overall survival rate was 53% at 1 year, 28% at 2 years, and 23% at 3 years. Grade 3 to 4 adverse events included hypertension (15%) and thrombosis (6%, including 4% with arterial thrombosis). Grade 3 or higher hemorrhage occurred in 11% of patients, including one fatal variceal bleed. Bevacizumab was associated with significant reductions in tumor enhancement by dynamic contrast-enhanced magnetic resonance imaging and reductions in circulating VEGF-A and stromal-derived factor-1 levels. Functional angiogenic activity was associated with VEGF-A levels in patient plasma.
Conclusion: We observed significant clinical and biologic activity for bevacizumab in nonmetastatic HCC and achieved the primary study end point. Serious bleeding complications occurred in 11% of patients. Further evaluation is warranted in carefully selected patients.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Figures
References
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. - PubMed
-
- El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: An update. Ann Intern Med. 2003;139:817–823. - PubMed
-
- Llovet J, Ricci S, Mazzaferro V, et al. Sorafenib improves survival in advanced hepatocellular carcinoma (HCC): Results of a phase III randomized placebo-controlled trial (SHARP trial) J Clin Oncol. 2007;25:1s. (suppl; abstr LBA1)
-
- Yamaguchi R, Yano H, Iemura A, et al. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

