Role of seed coat in imbibing soybean seeds observed by micro-magnetic resonance imaging
- PMID: 18565982
- PMCID: PMC2516911
- DOI: 10.1093/aob/mcn095
Role of seed coat in imbibing soybean seeds observed by micro-magnetic resonance imaging
Abstract
Background and aims: Imbibition of Japanese soybean (Glycine max) cultivars was studied using micro-magnetic resonance imaging (MRI) in order to elucidate the mechanism of soaking injury and the protective role of the seed coat.
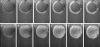
Methods: Time-lapse images during water uptake were acquired by the single-point imaging (SPI) method at 15-min intervals, for 20 h in the dry seed with seed coat, and for 2 h in seeds with the seed coat removed. The technique visualized water migration within the testa and demonstrated the distortion associated with cotyledon swelling during the very early stages of water uptake.
Key results: Water soon appeared in the testa and went around the dorsal surface of the seed from near the raphe, then migrated to the hilum region. An obvious protrusion was noted when water reached the hypocotyl and the radicle, followed by swelling of the cotyledons. A convex area was observed around the raphe with the enlargement of the seed. Water was always incorporated into the cotyledons from the abaxial surfaces, leading to swelling and generating a large air space between the adaxial surfaces. Water uptake greatly slowed, and the internal structures, veins and oil-accumulating tissues in the cotyledons developed after the seed stopped expanding. When the testa was removed from the dry seeds before imbibition, the cotyledons were severely damaged within 1.5 h of water uptake.
Conclusions: The activation of the water channel seemed unnecessary for water entry into soybean seeds, and the testa rapidly swelled with steeping in water. However, the testa did not regulate the water incorporation in itself, but rather the rate at which water encountered the hypocotyl, the radicle, and the cotyledons through the inner layer of the seed coat, and thus prevented the destruction of the seed tissues at the beginning of imbibition.
Figures








Similar articles
-
Water uptake by dry beans observed by micro-magnetic resonance imaging.Ann Bot. 2006 Sep;98(3):545-53. doi: 10.1093/aob/mcl145. Epub 2006 Jul 15. Ann Bot. 2006. PMID: 16845137 Free PMC article.
-
Patterns and kinetics of water uptake by soybean seeds.J Exp Bot. 2007;58(3):717-32. doi: 10.1093/jxb/erl244. Epub 2006 Dec 21. J Exp Bot. 2007. PMID: 17185739
-
Water uptake and oil distribution during imbibition of seeds of western white pine (Pinus monticola Dougl. ex D. Don) monitored in vivo using magnetic resonance imaging.Planta. 2005 Apr;221(1):17-27. doi: 10.1007/s00425-004-1426-z. Epub 2004 Dec 17. Planta. 2005. PMID: 15605241
-
The role of the testa during development and in establishment of dormancy of the legume seed.Front Plant Sci. 2014 Jul 17;5:351. doi: 10.3389/fpls.2014.00351. eCollection 2014. Front Plant Sci. 2014. PMID: 25101104 Free PMC article. Review.
-
The orchid seed coat: a developmental and functional perspective.Bot Stud. 2023 Sep 27;64(1):27. doi: 10.1186/s40529-023-00400-0. Bot Stud. 2023. PMID: 37755558 Free PMC article. Review.
Cited by
-
Water entry for the black locust (Robinia pseudoacacia L.) seeds observed by dedicated micro-magnetic resonance imaging.J Plant Res. 2016 Jul;129(4):667-673. doi: 10.1007/s10265-016-0823-2. Epub 2016 Apr 8. J Plant Res. 2016. PMID: 27059756
-
Investment in seed physical defence is associated with species' light requirement for regeneration and seed persistence: evidence from Macaranga species in Borneo.PLoS One. 2014 Jun 13;9(6):e99691. doi: 10.1371/journal.pone.0099691. eCollection 2014. PLoS One. 2014. PMID: 24927025 Free PMC article.
-
Seed germination of Agave species as influenced by substrate water potential.Biol Res. 2014 Apr 1;47(1):11. doi: 10.1186/0717-6287-47-11. Biol Res. 2014. PMID: 25027050 Free PMC article.
-
A Single-Nucleotide Polymorphism in an Endo-1,4-β-Glucanase Gene Controls Seed Coat Permeability in Soybean.PLoS One. 2015 Jun 3;10(6):e0128527. doi: 10.1371/journal.pone.0128527. eCollection 2015. PLoS One. 2015. PMID: 26039079 Free PMC article.
-
A Combined Comparative Transcriptomic, Metabolomic, and Anatomical Analyses of Two Key Domestication Traits: Pod Dehiscence and Seed Dormancy in Pea (Pisum sp.).Front Plant Sci. 2017 Apr 25;8:542. doi: 10.3389/fpls.2017.00542. eCollection 2017. Front Plant Sci. 2017. PMID: 28487704 Free PMC article.
References
-
- Ashworth EN, Obendorf RL. Imbibitional chilling injury in soybean axes: relationship to stelar lesions and seasonal environments. Agronomy Journal. 1980;72:923–928.
-
- Bemporad D, Luttmann C, Essex JW. Behaviour of small solutes and large drugs in a lipid bilayer from computer simulations. Biochimica et Biophysica Acta. 2005;1718:1–21. - PubMed
-
- Chamberlin MA, Horner HT, Palmer RG. Early endosperm, embryo, and ovule development in Glycine max (L.) Merr. International Journal of Plant Science. 1994;155:421–436.
-
- Dell B. Structure and function of the strophiolar plug in seeds of Albizia lophantha. American Journal of Botany. 1980;67:556–563.

