The presence of pacemaker HCN channels identifies theta rhythmic GABAergic neurons in the medial septum
- PMID: 18565991
- PMCID: PMC2538919
- DOI: 10.1113/jphysiol.2008.155242
The presence of pacemaker HCN channels identifies theta rhythmic GABAergic neurons in the medial septum
Abstract
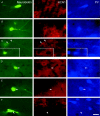
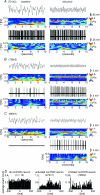
The medial septum (MS) is an indispensable component of the subcortical network which synchronizes the hippocampus at theta frequency during specific stages of information processing. GABAergic neurons exhibiting highly regular firing coupled to the hippocampal theta rhythm are thought to form the core of the MS rhythm-generating network. In recent studies the hyperpolarization-activated, cyclic nucleotide-gated non-selective cation (HCN) channel was shown to participate in theta synchronization of the medial septum. Here, we tested the hypothesis that HCN channel expression correlates with theta modulated firing behaviour of MS neurons by a combined anatomical and electrophysiological approach. HCN-expressing neurons represented a subpopulation of GABAergic cells in the MS partly overlapping with parvalbumin (PV)-containing neurons. Rhythmic firing in the theta frequency range was characteristic of all HCN-expressing neurons. In contrast, only a minority of HCN-negative cells displayed theta related activity. All HCN cells had tight phase coupling to hippocampal theta waves. As a group, PV-expressing HCN neurons had a marked bimodal phase distribution, whereas PV-immunonegative HCN neurons did not show group-level phase preference despite significant individual phase coupling. Microiontophoretic blockade of HCN channels resulted in the reduction of discharge frequency, but theta rhythmic firing was perturbed only in a few cases. Our data imply that HCN-expressing GABAergic neurons provide rhythmic drive in all phases of the hippocampal theta activity. In most MS theta cells rhythm genesis is apparently determined by interactions at the level of the network rather than by the pacemaking property of HCN channels alone.
Figures





References
-
- Alonso A, Gaztelu JM, Buno W, Jr, Garcia-Austt E. Cross-correlation analysis of septohippocampal neurons during theta rhythm. Brain Res. 1987;413:135–146. - PubMed
-
- Alreja M. Excitatory actions of serotonin on GABAergic neurons of the medial septum and diagonal band of Broca. Synapse. 1996;22:15–27. - PubMed
-
- Barrenechea C, Pedemonte M, Nunez A, Garcia-Austt E. In vivo intracellular recordings of medial septal and diagonal band of Broca neurons: relationships with theta rhythm. Exp Brain Res. 1995;103:31–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

