Fetal exposure to high-avidity TCR ligand enhances expansion of peripheral T regulatory cells
- PMID: 18566371
- PMCID: PMC2678955
- DOI: 10.4049/jimmunol.181.1.73
Fetal exposure to high-avidity TCR ligand enhances expansion of peripheral T regulatory cells
Abstract
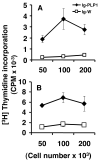
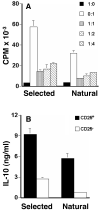
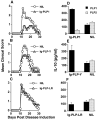
Lately, it has become clear that regulatory T cells (Tregs) play a major role in the maintenance of peripheral tolerance and control of autoimmunity. Despite these critical functions, the process underlying the development of Tregs remains largely undefined. Herein, altered peptide ligand (APL) variants derived from the proteolipid protein-1 (PLP1) epitope were expressed on immunoglobulins (Igs) and the resulting Ig-APLs were used to deliver the APLs from mother to fetus through the maternal placenta to influence thymic T cell selection. This delivery system was then adapted to the SJL/J mouse, a strain that expresses only the DM20 form of PLP, which lacks the dominant PLP1 epitope in the thymus during fetal and neonatal development. This model, which restores thymic T cell selection for PLP1, was then used to determine whether affinity plays a role in the development of Tregs. The findings show that fetal exposure to low-affinity peptide ligand was unable to drive development of Tregs while variants with higher affinity to the TCR resulted in significant seeding of the periphery with mature, naive Tregs. Thus, contrary to pathogenic T cells, Tregs require avid TCR-ligand interaction to undergo thymic development and maturation.
Figures





References
-
- Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. - PubMed
-
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self tolerance maintained by T cells expressing IL-2 receptor alpha chains (CD25). Breakdown of a single mechanism of self tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. - PubMed
-
- Shevach EM. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 2000;18:423–449. - PubMed
-
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

