An age-old paradigm challenged: old baboons generate vigorous humoral immune responses to LcrV, a plague antigen
- PMID: 18566375
- PMCID: PMC3663140
- DOI: 10.4049/jimmunol.181.1.109
An age-old paradigm challenged: old baboons generate vigorous humoral immune responses to LcrV, a plague antigen
Abstract
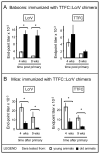
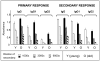
Immune senescence in the elderly results in decreased immunity with a concomitant increase in susceptibility to infection and diminished efficacy of vaccination. Nonhuman primate models have proven critical for testing of vaccines and therapeutics in the general population, but a model using old animals has not been established. Toward that end, immunity to LcrV, a protective Ag from Yersinia pestis, was tested in young and old baboons. Surprisingly, there was no age-associated loss in immune competence; LcrV elicited high-titer, protective Ab responses in the older individuals. The primary responses in the younger baboons were lower, but they did show boosting upon secondary immunization to the levels achieved in the old animals. The LcrV Ag was also tested in mice and, as expected, age-associated loss of immunity was seen; older animals responded with lower-titer Abs and, as a result, were more susceptible to Yersinia challenge. Thus, although age-related loss in immune function has been observed in humans, rodents, and some nonhuman primates, baboons appear to be unusual; they age without losing immune competence.
Figures



References
-
- Gavazzi G, Krause KH. Ageing and Infection. Lancet Infectious Dis. 2002;2:659–666. - PubMed
-
- Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes and Infect. 2001;3:851–857. - PubMed
-
- Webster RG. Immunity to influenze in the elderly. Vaccine. 2000;18:1686–1689. - PubMed
-
- Hakim FT, Flomerfelt FA, Boyiadzis M, Gress RE. Aging, immunity and cancer. Current Opinion in Immunol. 2004;16:151–156. - PubMed
-
- Oxman MN, Levin MJJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang Hongyuan J, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh S-S, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan ISF, Wang WWB, Annunziato PW, Silber JL. A Vaccine to Prevent Herpes Zoster and Postherpetic Neuralgia in Older Adults. New England Journal of Med. 2005;352:2271–2284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

