Activation of the JAK/STAT-1 signaling pathway by IFN-gamma can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG
- PMID: 18566411
- PMCID: PMC2667120
- DOI: 10.4049/jimmunol.181.1.449
Activation of the JAK/STAT-1 signaling pathway by IFN-gamma can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG
Abstract
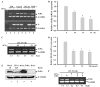
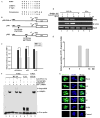
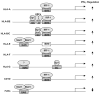
Expression of many MHC genes is enhanced at the transcriptional or posttranscriptional level following exposure to the cytokine IFN-gamma. However, in this study we found that IFN-gamma down-regulated the constitutive expression of the neonatal Fc receptor (FcRn), an MHC class I-related molecule that functions to transport maternal IgG and protect IgG and albumin from degradation. Epithelial cell, macrophage-like THP-1 cell, and freshly isolated human PBMC exposure to IFN-gamma resulted in a significant decrease of FcRn expression as assessed by real-time RT-PCR and Western blotting. The down-regulation of FcRn was not caused by apoptosis or the instability of FcRn mRNA. Chromatin immunoprecipitation and gel mobility shift assays showed that STAT-1 bound to an IFN-gamma activation site in the human FcRn promoter region. Luciferase expression from an FcRn promoter-luciferase reporter gene construct was not altered in JAK1- and STAT-1-deficient cells following exposure to IFN-gamma, whereas expression of JAK1 or STAT-1 protein restored the IFN-gamma inhibitory effect on luciferase activity. The repressive effect of IFN-gamma on the FcRn promoter was selectively reversed or blocked by mutations of the core nucleotides in the IFN-gamma activation site sequence and by overexpression of the STAT-1 inhibitor PIAS1 or the dominant negative phospho-STAT-1 mutations at Tyr-701 and/or Ser-727 residues. Furthermore, STAT-1 might down-regulate FcRn transcription through sequestering the transcriptional coactivator CREB binding protein/p300. Functionally, IFN-gamma stimulation dampened bidirectional transport of IgG across a polarized Calu-3 lung epithelial monolayer. Taken together, our results indicate that the JAK/STAT-1 signaling pathway was necessary and sufficient to mediate the down-regulation of FcRn gene expression by IFN-gamma.
Figures




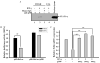

References
-
- Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10:1289–1298. - PubMed
-
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

