Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection
- PMID: 18566421
- PMCID: PMC2668691
- DOI: 10.4049/jimmunol.181.1.546
Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection
Abstract
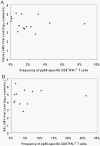
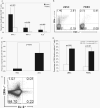
Acquisition of T cell responses during primary CMV infection in lung transplant recipients (LTRs) appear critical for host defense and allograft durability, with increased mortality in donor+/recipient- (D+R-) individuals. In 15 D+R- LTRs studied, acute primary CMV infection was characterized by viremia in the presence or absence of pneumonitis, with viral loads higher in the lung airways/allograft compared with the blood. A striking influx of CD8+ T cells into the lung airways/allograft was observed, with inversion of the CD4+:CD8+ T cell ratio. De novo CMV-specific CD8+ effector frequencies in response to pooled peptides of pp65 were strikingly higher in lung mononuclear cells compared with the PBMC and predominated over IE1-specific responses and CD4+ effector responses in both compartments. The frequencies of pp65-specific cytokine responses were significantly higher in lung mononuclear cells compared with PBMC and demonstrated marked contraction with long-term persistence of effector memory CD8+ T cells in the lung airways following primary infection. CMV-tetramer+CD8+ T cells from PBMC were CD45RA- during viremia and transitioned to CD45RA+ following resolution. In contrast, CMV-specific CD8+ effectors in the lung airways/allograft maintained a CD45RA- phenotype during transition from acute into chronic infection. Together, these data reveal differential CMV-specific CD8+ effector frequencies, immunodominance, and polyfunctional cytokine responses predominating in the lung airways/allograft compared with the blood during acute primary infection. Moreover, we show intercompartmental phenotypic differences in CMV-specific memory responses during the transition to chronic infection.
Figures





References
-
- Fishman JA, Rubin RH. Infection in organ-transplant recipients. N. Engl. J. Med. 1998;338:1741–1751. - PubMed
-
- Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am. J. Respir. Crit. Care Med. 2004;170:22–48. - PubMed
-
- Rubin RH. Cytomegalovirus in solid organ transplantation. Transpl. Infect. Dis. 2001;3(Suppl 2):1–5. - PubMed
-
- Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J. Heart Lung Transplant. 2002;21:297–310. - PubMed
-
- Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064–2068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

