Stress-protective signalling of prion protein is corrupted by scrapie prions
- PMID: 18566584
- PMCID: PMC2486277
- DOI: 10.1038/emboj.2008.122
Stress-protective signalling of prion protein is corrupted by scrapie prions
Abstract
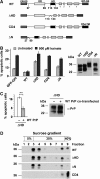
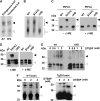
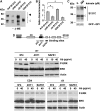
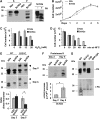
Studies in transgenic mice revealed that neurodegeneration induced by scrapie prion (PrP(Sc)) propagation is dependent on neuronal expression of the cellular prion protein PrP(C). On the other hand, there is evidence that PrP(C) itself has a stress-protective activity. Here, we show that the toxic activity of PrP(Sc) and the protective activity of PrP(C) are interconnected. With a novel co-cultivation assay, we demonstrate that PrP(Sc) can induce apoptotic signalling in PrP(C)-expressing cells. However, cells expressing PrP mutants with an impaired stress-protective activity were resistant to PrP(Sc)-induced toxicity. We also show that the internal hydrophobic domain promotes dimer formation of PrP and that dimerization of PrP is linked to its stress-protective activity. PrP mutants defective in dimer formation did not confer enhanced stress tolerance. Moreover, in chronically scrapie-infected neuroblastoma cells the amount of PrP(C) dimers inversely correlated with the amount of PrP(Sc) and the resistance of the cells to various stress conditions. Our results provide new insight into the mechanism of PrP(C)-mediated neuroprotection and indicate that pathological PrP conformers abuse PrP(C)-dependent pathways for apoptotic signalling.
Figures
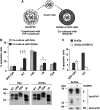
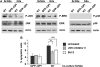

References
-
- Aguzzi A, Polymenidou M (2004) Mammalian prion biology: one century of evolving concepts. Cell 116: 313–327 - PubMed
-
- Borchelt DR, Taraboulos A, Prusiner SB (1992) Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem 267: 16188–16199 - PubMed
-
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A (1996) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379: 339–343 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

