The structure of P-TEFb (CDK9/cyclin T1), its complex with flavopiridol and regulation by phosphorylation
- PMID: 18566585
- PMCID: PMC2486423
- DOI: 10.1038/emboj.2008.121
The structure of P-TEFb (CDK9/cyclin T1), its complex with flavopiridol and regulation by phosphorylation
Abstract
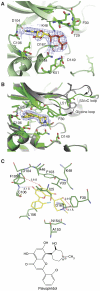
The positive transcription elongation factor b (P-TEFb) (CDK9/cyclin T (CycT)) promotes mRNA transcriptional elongation through phosphorylation of elongation repressors and RNA polymerase II. To understand the regulation of a transcriptional CDK by its cognate cyclin, we have determined the structures of the CDK9/CycT1 and free cyclin T2. There are distinct differences between CDK9/CycT1 and the cell cycle CDK CDK2/CycA manifested by a relative rotation of 26 degrees of CycT1 with respect to the CDK, showing for the first time plasticity in CDK cyclin interactions. The CDK9/CycT1 interface is relatively sparse but retains some core CDK-cyclin interactions. The CycT1 C-terminal helix shows flexibility that may be important for the interaction of this region with HIV TAT and HEXIM. Flavopiridol, an anticancer drug in phase II clinical trials, binds to the ATP site of CDK9 inducing unanticipated structural changes that bury the inhibitor. CDK9 activity and recognition of regulatory proteins are governed by autophosphorylation. We show that CDK9/CycT1 autophosphorylates on Thr186 in the activation segment and three C-terminal phosphorylation sites. Autophosphorylation on all sites occurs in cis.
Figures





References
-
- Brown NR, Lowe ED, Petri E, Skamnaki V, Antrobus R, Johnson LN (2007) Cyclin B and cyclin A confer different substrate recognition properties on CDK2. Cell Cycle 6: 1350–1359 - PubMed
-
- Brown NR, Noble ME, Endicott JA, Garman EF, Wakatsuki S, Mitchell E, Rasmussen B, Hunt T, Johnson LN (1995) The crystal structure of cyclin A. Structure 3: 1235–1247 - PubMed
-
- Brown NR, Noble ME, Endicott JA, Johnson LN (1999) The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat Cell Biol 1: 438–443 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

