The bioinorganic chemistry and associated immunology of chronic beryllium disease
- PMID: 18566702
- PMCID: PMC4793722
- DOI: 10.1039/b718746g
The bioinorganic chemistry and associated immunology of chronic beryllium disease
Abstract
Chronic beryllium disease (CBD) is a debilitating, incurable, and often fatal disease that is caused by the inhalation of beryllium particulates. The growing use of beryllium in the modern world, in products ranging from computers to dental prosthetics (390 tons of beryllium in the US in the year 2000) necessitates a molecular based understanding of the disease in order to prevent and cure CBD. We have investigated the molecular basis of CBD at Los Alamos National Laboratory during the past six years, employing a multidisciplinary approach of bioinorganic chemistry and immunology. The results of this work, including speciation, inhalation and dissolution, and immunology will be discussed.
Figures





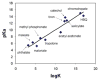

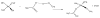
References
-
- Everest DA. Chemistry of Beryllium. ch 1 Elsevier; New York: 1964.
-
- Taylor TP, Ding M, Ehler DS, Foreman TM, Kaszuba JP, Sauer NN. J Environ Sci Health, Part A. 2003;38:439. - PubMed
-
- Bellamy RG, Hill NA. In: Extraction and Metallurgy of Uranium, Thorium, and Beryllium. Dunworth JV, editor. Macmillan; New York: 1963. pp. 1–17.
-
- Wataha JC. J Prosthet Dent. 2000;83:223. - PubMed
-
- Parsonage T. Mater Sci Technol. 2000;16:732.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

