CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta
- PMID: 18566967
- PMCID: PMC2671575
- DOI: 10.1002/humu.20799
CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta
Abstract
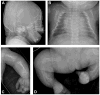
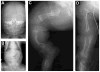
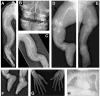
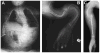
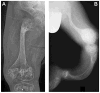
Autosomal dominant osteogenesis imperfecta (OI) is caused by mutations in the genes (COL1A1 or COL1A2) encoding the chains of type I collagen. Recently, dysregulation of hydroxylation of a single proline residue at position 986 of both the triple-helical domains of type I collagen alpha1(I) and type II collagen alpha1(II) chains has been implicated in the pathogenesis of recessive forms of OI. Two proteins, cartilage-associated protein (CRTAP) and prolyl-3-hydroxylase-1 (P3H1, encoded by the LEPRE1 gene) form a complex that performs the hydroxylation and brings the prolyl cis-trans isomerase cyclophilin-B (CYPB) to the unfolded collagen. In our screen of 78 subjects diagnosed with OI type II or III, we identified three probands with mutations in CRTAP and 16 with mutations in LEPRE1. The latter group includes a mutation in patients from the Irish Traveller population, a genetically isolated community with increased incidence of OI. The clinical features resulting from CRTAP or LEPRE1 loss of function mutations were difficult to distinguish at birth. Infants in both groups had multiple fractures, decreased bone modeling (affecting especially the femurs), and extremely low bone mineral density. Interestingly, "popcorn" epiphyses may reflect underlying cartilaginous and bone dysplasia in this form of OI. These results expand the range of CRTAP/LEPRE1 mutations that result in recessive OI and emphasize the importance of distinguishing recurrence of severe OI of recessive inheritance from those that result from parental germline mosaicism for COL1A1 or COL1A2 mutations.
Figures

References
-
- Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355(26):2757–64. - PMC - PubMed
-
- Barry J, Kirke P. Congenital anomalies in the Irish traveller community. Ir Med J. 1997;90(6):233–4. - PubMed
-
- Byers PH, Cole WG. Osteogenesis Imperfecta. In: Steinmann BU, editor. Connective tissue and it heritable disorders: molecular, genetic, and medical aspects. New York: Wiley-Liss; 2002. pp. 285–430.
-
- Cabral WA, Barnes AM, Porter F, Marini JC. Carrier frequency of recurring mutation causing severe/lethal recessive type VIII osteogenesis imperfecta in African-Americans; 2007a October 23–27; San Diego.
-
- Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, Leikin S, Makareeva E, Kuznetsova NV, Rosenbaum KN, Tifft CJ, Bulas DI, Kozma C, Smith PA, Eyre DR, Marini JC. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007b;39(3):359–65. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

