Efficacy of Artequick versus artesunate-mefloquine in the treatment of acute uncomplicated falciparum malaria in Thailand
- PMID: 18567436
- PMCID: PMC3129605
Efficacy of Artequick versus artesunate-mefloquine in the treatment of acute uncomplicated falciparum malaria in Thailand
Abstract
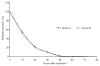
To determine the efficacy, safety and tolerability of an alternative short-course, artemisinin-based combination therapy for acute uncomplicated Plasmodium falciparum malaria, we compared Artequick--a fixed-dosed combination of artemisinin (80 mg), piperaquine (400 mg), and primaquine (4 mg), per tablet--with a standard regimen of artesunate-mefloquine. A total of 130 patients were randomly assigned to treatment with an orally administered, once-daily, 3-day regimen of either Artequick (Group A: 3.2 mg/Kg/day of artemisinin, 16 mg/Kg/day of piperaquine, and 0.16 mg/Kg/day of primaquine) or artesunate-mefloquine (Group B: artesunate, 4 mg/Kg/day, with mefloquine, 8 mg/Kg/day). Patients receiving each regimen had a rapid clinical and parasitological response. All treatments were well tolerated, and no serious adverse effects occurred. No significant differences were found in fever- and parasite-clearance times between the two study groups. The 28-day cure rates were similarly high, at 98.5% and 100%, in groups A and B, respectively. We conclude that Artequick was as effective and well tolerated as artesunate-mefloquine and could be used as an alternative treatment for multidrug-resistant Plasmodium falciparum malaria in Southeast Asia.
Figures
References
-
- Ashley EA, White NJ. Artemisinin-based combinations. Curr Opin Infect Dis. 2005;18:531–6. - PubMed
-
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. - PubMed
-
- Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: a resurgent antimalarial drug. Drugs. 2005;65:75–87. - PubMed
-
- Denis MB, Davis TM, Hewitt S, et al. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin Infect Dis. 2002;35:1469–76. - PubMed
-
- Hien TT, Dolecek C, Mai PP. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomized clinical trial. Lancet. 2004;363:18–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources