Membrane composition modulates prestin-associated charge movement
- PMID: 18567583
- PMCID: PMC2504877
- DOI: 10.1074/jbc.M803722200
Membrane composition modulates prestin-associated charge movement
Abstract
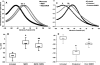
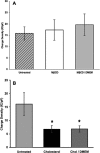
The lateral membrane of the cochlear outer hair cell (OHC) is the site of a membrane-based motor that powers OHC electromotility, enabling amplification and fine-tuning of auditory signals. The OHC membrane protein prestin plays a central role in this process. We have previously shown that membrane cholesterol modulates the peak voltage of prestin-associated nonlinear capacitance in vivo and in vitro. The present study explores the effects of membrane cholesterol and docosahexaenoic acid content on the peak and magnitude of prestin-associated charge movement in a human embryonic kidney (HEK 293) cell model. Increasing membrane cholesterol results in a hyperpolarizing shift in the peak voltage of the nonlinear capacitance (Vpkc) and a decrease in the total charge movement. Both measures depend linearly on membrane cholesterol concentration. Incubation of cholesterol-loaded cells in cholesterol-free media partially restores the Vpkc toward normal values but does not have a compensatory effect on the total charge movement. Decreasing membrane cholesterol results in a depolarizing shift in Vpkc that is restored toward normal values upon incubation in cholesterol-free media. However, cholesterol depletion does not alter the magnitude of charge movement. In contrast, increasing membrane docosahexaenoic acid results in a hyperpolarizing shift in Vpkc that is accompanied by an increase in total charge movement. Our results quantify the relation between membrane cholesterol concentration and prestin-associated charge movement and enhance our understanding of how membrane composition modulates prestin function.
Figures



Similar articles
-
Membrane prestin expression correlates with the magnitude of prestin-associated charge movement.Hear Res. 2016 Sep;339:50-9. doi: 10.1016/j.heares.2016.05.016. Epub 2016 Jun 1. Hear Res. 2016. PMID: 27262187 Free PMC article.
-
Effects of membrane potential and tension on prestin, the outer hair cell lateral membrane motor protein.J Physiol. 2001 Mar 15;531(Pt 3):661-6. doi: 10.1111/j.1469-7793.2001.0661h.x. J Physiol. 2001. PMID: 11251048 Free PMC article.
-
Tuning of the outer hair cell motor by membrane cholesterol.J Biol Chem. 2007 Dec 14;282(50):36659-70. doi: 10.1074/jbc.M705078200. Epub 2007 Oct 12. J Biol Chem. 2007. PMID: 17933870 Free PMC article.
-
Cochlear amplification, outer hair cells and prestin.Curr Opin Neurobiol. 2008 Aug;18(4):370-6. doi: 10.1016/j.conb.2008.08.016. Epub 2008 Oct 4. Curr Opin Neurobiol. 2008. PMID: 18809494 Free PMC article. Review.
-
Prestin and the cochlear amplifier.J Physiol. 2006 Oct 1;576(Pt 1):37-42. doi: 10.1113/jphysiol.2006.114652. Epub 2006 Jul 27. J Physiol. 2006. PMID: 16873410 Free PMC article. Review.
Cited by
-
A novel theoretical framework reveals more than one voltage-sensing pathway in the lateral membrane of outer hair cells.J Gen Physiol. 2020 Jul 6;152(7):e201912447. doi: 10.1085/jgp.201912447. J Gen Physiol. 2020. PMID: 32384538 Free PMC article.
-
Lipid lateral mobility in cochlear outer hair cells: regional differences and regulation by cholesterol.J Assoc Res Otolaryngol. 2009 Sep;10(3):383-96. doi: 10.1007/s10162-009-0171-1. Epub 2009 Jun 11. J Assoc Res Otolaryngol. 2009. PMID: 19517190 Free PMC article.
-
Biophysical mechanisms underlying outer hair cell loss associated with a shortened tectorial membrane.J Assoc Res Otolaryngol. 2011 Oct;12(5):577-94. doi: 10.1007/s10162-011-0269-0. Epub 2011 May 13. J Assoc Res Otolaryngol. 2011. PMID: 21567249 Free PMC article.
-
Glycosylation regulates prestin cellular activity.J Assoc Res Otolaryngol. 2010 Mar;11(1):39-51. doi: 10.1007/s10162-009-0196-5. Epub 2009 Nov 7. J Assoc Res Otolaryngol. 2010. PMID: 19898896 Free PMC article.
-
Diflunisal inhibits prestin by chloride-dependent mechanism.PLoS One. 2017 Aug 17;12(8):e0183046. doi: 10.1371/journal.pone.0183046. eCollection 2017. PLoS One. 2017. PMID: 28817613 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

