Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state
- PMID: 18567661
- PMCID: PMC2519390
- DOI: 10.1128/JB.01919-07
Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state
Abstract
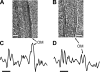
The cell envelope of mycobacteria, which include the causative agents of tuberculosis and leprosy, is crucial for their success as pathogens. Despite a continued strong emphasis on identifying the multiple chemical components of this envelope, it has proven difficult to combine its components into a comprehensive structural model, primarily because the available ultrastructural data rely on conventional electron microscopy embedding and sectioning, which are known to induce artifacts. The existence of an outer membrane bilayer has long been postulated but has never been directly observed by electron microscopy of ultrathin sections. Here we have used cryo-electron microscopy of vitreous sections (CEMOVIS) to perform a detailed ultrastructural analysis of three species belonging to the Corynebacterineae suborder, namely, Mycobacterium bovis BCG, Mycobacterium smegmatis, and Corynebacterium glutamicum, in their native state. We provide new information that accurately describes the different layers of the mycobacterial cell envelope and challenges current models of the organization of its components. We show a direct visualization of an outer membrane, analogous to that found in gram-negative bacteria, in the three bacterial species examined. Furthermore, we demonstrate that mycolic acids, the hallmark of mycobacteria and related genera, are essential for the formation of this outer membrane. In addition, a granular layer and a low-density zone typifying the periplasmic space of gram-positive bacteria are apparent in CEMOVIS images of mycobacteria and corynebacteria. Based on our observations, a model of the organization of the lipids in the outer membrane is proposed. The architecture we describe should serve as a reference for future studies to relate the structure of the mycobacterial cell envelope to its function.
Figures




References
-
- Al-Amoudi, A., D. Studer, and J. Dubochet. 2005. Cutting artefacts and cutting process in vitreous sections for cryo-electron microscopy. J. Struct. Biol. 150109-121. - PubMed
-
- Alderwick, L. J., E. Radmacher, M. Seidel, R. Gande, P. G. Hitchen, H. R. Morris, A. Dell, H. Sahm, L. Eggeling, and G. S. Besra. 2005. Deletion of Cg-emb in Corynebacterianeae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J. Biol. Chem. 28032362-32371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

