Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling
- PMID: 18567706
- PMCID: PMC2519205
- DOI: 10.1152/ajpheart.00377.2008
Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling
Abstract
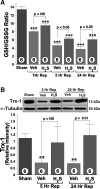
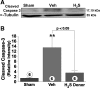
Hydrogen sulfide (H(2)S) is an endogenously produced gaseous signaling molecule with diverse physiological activity. The potential protective effects of H(2)S have not been evaluated in the liver. The purpose of the current study was to investigate if H(2)S could afford hepatoprotection in a murine model of hepatic ischemia-reperfusion (I/R) injury. Hepatic injury was achieved by subjecting mice to 60 min of ischemia followed by 5 h of reperfusion. H(2)S donor (IK1001) or vehicle were administered 5 min before reperfusion. H(2)S attenuated the elevation in serum alanine aminotransferase (ALT) by 68.6% and aspartate aminotransferase (AST) by 70.8% compared with vehicle group. H(2)S-mediated cytoprotection was associated with an improved balance between reduced glutathione (GSH) vs. oxidized glutathione (GSSG), an attenuated formation of lipid hydroperoxides, and an increased expression of thioredoxin-1 (Trx-1). Furthermore, H(2)S inhibited the progression of apoptosis after I/R injury by increasing the protein expression of heat shock protein (HSP-90) and Bcl-2. These results indicate that H(2)S protects the murine liver against I/R injury through an upregulation of intracellular antioxidant and antiapoptotic signaling pathways.
Figures

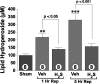
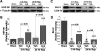
References
-
- Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006. - PubMed
-
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004. - PubMed
-
- Dias S, Shmelkov SV, Lam G, Rafii S. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood 99: 2532–2540, 2002. - PubMed
-
- Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 291: H2980–H2986, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

