The binding sites for cocaine and dopamine in the dopamine transporter overlap
- PMID: 18568020
- PMCID: PMC2692229
- DOI: 10.1038/nn.2146
The binding sites for cocaine and dopamine in the dopamine transporter overlap
Abstract
Cocaine is a widely abused substance with psychostimulant effects that are attributed to inhibition of the dopamine transporter (DAT). We present molecular models for DAT binding of cocaine and cocaine analogs constructed from the high-resolution structure of the bacterial transporter homolog LeuT. Our models suggest that the binding site for cocaine and cocaine analogs is deeply buried between transmembrane segments 1, 3, 6 and 8, and overlaps with the binding sites for the substrates dopamine and amphetamine, as well as for benztropine-like DAT inhibitors. We validated our models by detailed mutagenesis and by trapping the radiolabeled cocaine analog [3H]CFT in the transporter, either by cross-linking engineered cysteines or with an engineered Zn2+-binding site that was situated extracellularly to the predicted common binding pocket. Our data demonstrate the molecular basis for the competitive inhibition of dopamine transport by cocaine.
Figures




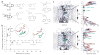
References
-
- Aguayo LG, et al. Historical and current perspectives of neuroactive compounds derived from Latin America. Mini Rev Med Chem. 2006;6:997–1008. - PubMed
-
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. - PubMed
-
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

