Rapid and modifiable neurotransmitter receptor dynamics at a neuronal synapse in vivo
- PMID: 18568021
- PMCID: PMC2700019
- DOI: 10.1038/nn.2145
Rapid and modifiable neurotransmitter receptor dynamics at a neuronal synapse in vivo
Abstract
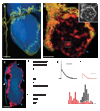
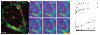
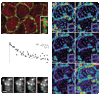
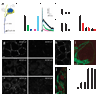
Synaptic plasticity underlies the adaptability of the mammalian brain, but has been difficult to study in living animals. Here we imaged the synapses between pre- and postganglionic neurons in the mouse submandibular ganglion in vivo, focusing on the mechanisms that maintain and regulate neurotransmitter receptor density at postsynaptic sites. Normally, synaptic receptor densities were maintained by rapid exchange of receptors with nonsynaptic regions (over minutes) and by continual turnover of cell surface receptors (over hours). However, after ganglion cell axons were crushed, synaptic receptors showed greater lateral mobility and there was a precipitous decline in insertion. These changes led to near-complete loss of synaptic receptors and synaptic depression. Disappearance of postsynaptic spines and presynaptic terminals followed this acute synaptic depression. Therefore, neurotransmitter receptor dynamism associated with rapid changes in synaptic efficacy precedes long-lasting structural changes in synaptic connectivity.
Figures



References
-
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. - PubMed
-
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extra-synaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. - PubMed
-
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. - PubMed
-
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. - PubMed
-
- Lu W, et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

