Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling
- PMID: 18570116
- PMCID: PMC2570775
- DOI: 10.1080/14767050802045848
Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling
Abstract
Objective: Examination of the amniotic fluid proteome has been used to identify biomarkers for intra-amniotic inflammation as well as those that may be useful in predicting the outcome of preterm labor. The purpose of this study was to combine a novel computational method of pattern discovery with mass spectrometric proteomic profiling of amniotic fluid to discover biomarkers of intra-amniotic infection/inflammation (IAI).
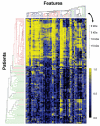
Methods: This cross-sectional study included patients with spontaneous preterm labor and intact membranes who delivered at term (n = 59) and those who delivered preterm with IAI (n = 60). Proteomic profiling was performed using surface-enhanced laser desorption/ionization (SELDI) mass spectrometry. A proteomic profile was acquired through multiple simultaneous SELDI conditions, which were combined in a single proteomic 'fingerprint' using a novel computational approach. Classification of patients based on their associated surface-enhanced laser desorption/ionization-time of flight (SELDI-TOF) mass spectra as belonging to either the class of individuals with preterm delivery with IAI or term delivery was accomplished by constructing an empirical model. The first phase in the construction of this empirical model involved the selection of adjustable parameters utilizing a training/testing subset of data. The second phase tested the generalization of the model by utilizing a blinded validation set of patients who were not employed in parameter selection.
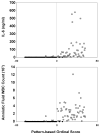
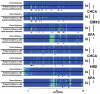
Results: Gestational age at amniocentesis was not significantly different between the groups. Thirty-nine unique mass spectrometric peaks discriminated patients with preterm labor/delivery with IAI from those with preterm labor and term delivery. In the testing/training dataset, the classification accuracies (averaged over 100 random draws) were: 91.4% (40.2/44) for patients with preterm delivery with IAI, and 91.2% (40.1/44) for term delivery. The overall accuracy of the classification of patients in the validation dataset was 90.3% (28/31).
Conclusions: Proteomic analysis of amniotic fluid allowed the identification of mass spectrometry features, which can distinguish patients with preterm labor with IAI from those with preterm labor without inflammation or infection who subsequently delivered at term.
Figures



Similar articles
-
Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers.JAMA. 2004 Jul 28;292(4):462-9. doi: 10.1001/jama.292.4.462. JAMA. 2004. PMID: 15280344
-
Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry.J Matern Fetal Neonatal Med. 2008 Oct;21(10):697-713. doi: 10.1080/14767050802053289. J Matern Fetal Neonatal Med. 2008. PMID: 19012186 Free PMC article.
-
Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery.J Matern Fetal Neonatal Med. 2010 Dec;23(12):1344-59. doi: 10.3109/14767058.2010.482618. Epub 2010 May 26. J Matern Fetal Neonatal Med. 2010. PMID: 20504069 Free PMC article.
-
Noninvasive Sampling of the Intrauterine Environment in Women with Preterm Labor and Intact Membranes.Fetal Diagn Ther. 2018;43(4):241-249. doi: 10.1159/000480232. Epub 2017 Oct 28. Fetal Diagn Ther. 2018. PMID: 29080890 Review.
-
The immunobiology of preterm labor and birth: intra-amniotic inflammation or breakdown of maternal-fetal homeostasis.Reproduction. 2022 Jun 20;164(2):R11-R45. doi: 10.1530/REP-22-0046. Reproduction. 2022. PMID: 35559791 Free PMC article. Review.
Cited by
-
Mass spectrometry-based proteomics for pre-eclampsia and preterm birth.Int J Mol Sci. 2015 May 14;16(5):10952-85. doi: 10.3390/ijms160510952. Int J Mol Sci. 2015. PMID: 26006232 Free PMC article. Review.
-
An overview of racial disparities in preterm birth rates: caused by infection or inflammatory response?Acta Obstet Gynecol Scand. 2011 Dec;90(12):1325-31. doi: 10.1111/j.1600-0412.2011.01135.x. Epub 2011 May 26. Acta Obstet Gynecol Scand. 2011. PMID: 21615712 Free PMC article. Review.
-
The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes.J Matern Fetal Neonatal Med. 2019 Feb;32(4):527-541. doi: 10.1080/14767058.2017.1384460. Epub 2017 Oct 11. J Matern Fetal Neonatal Med. 2019. PMID: 29020827 Free PMC article.
-
Characterization of Visceral and Subcutaneous Adipose Tissue Transcriptome and Biological Pathways in Pregnant and Non-Pregnant Women: Evidence for Pregnancy-Related Regional-Specific Differences in Adipose Tissue.PLoS One. 2015 Dec 4;10(12):e0143779. doi: 10.1371/journal.pone.0143779. eCollection 2015. PLoS One. 2015. PMID: 26636677 Free PMC article. Clinical Trial.
-
Characterization of visceral and subcutaneous adipose tissue transcriptome in pregnant women with and without spontaneous labor at term: implication of alternative splicing in the metabolic adaptations of adipose tissue to parturition.J Perinat Med. 2016 Oct 1;44(7):813-835. doi: 10.1515/jpm-2015-0259. J Perinat Med. 2016. PMID: 26994472 Free PMC article.
References
-
- Hack M, Fanaroff AA. Outcomes of extremely immature infants--a perinatal dilemma. N.Engl.J Med. 1993;329:1649–1650. - PubMed
-
- Dammann O, Leviton A, Gappa M, Dammann CE. Lung and brain damage in preterm newborns, and their association with gestational age, prematurity subgroup, infection/inflammation and long term outcome. BJOG. 2005;112(Suppl 1):4–9. - PubMed
-
- Leavitt RP, Green NS, Katz M. Current and future directions of research into prematurity: report of the symposium on prematurity held on 21-22 November 2005. Pediatr.Res. 2006;60:777–780. - PubMed
-
- Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118:1566–1573. - PubMed
-
- Moutquin JM. Classification and heterogeneity of preterm birth. BJOG. 2003;110(Suppl 20):30–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
