Source estimates for MEG/EEG visual evoked responses constrained by multiple, retinotopically-mapped stimulus locations
- PMID: 18570197
- PMCID: PMC2754810
- DOI: 10.1002/hbm.20597
Source estimates for MEG/EEG visual evoked responses constrained by multiple, retinotopically-mapped stimulus locations
Abstract
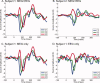
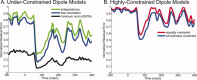
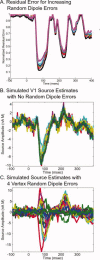
Studying the human visual system with high temporal resolution is a significant challenge due to the limitations of the available, noninvasive measurement tools. MEG and EEG provide the millisecond temporal resolution necessary for answering questions about intracortical communication involved in visual processing, but source estimation is ill-posed and unreliable when multiple; simultaneously active areas are located close together. To address this problem, we have developed a retinotopy-constrained source estimation method to calculate the time courses of activation in multiple visual areas. Source estimation was disambiguated by: (1) fixing MEG/EEG generator locations and orientations based on fMRI retinotopy and surface tessellations constructed from high-resolution MRI images; and (2) solving for many visual field locations simultaneously in MEG/EEG responses, assuming source current amplitudes to be constant or varying smoothly across the visual field. Because of these constraints on the solutions, estimated source waveforms become less sensitive to sensor noise or random errors in the specification of the retinotopic dipole models. We demonstrate the feasibility of this method and discuss future applications such as studying the timing of attentional modulation in individual visual areas.
2008 Wiley-Liss, Inc.
Figures







References
-
- Ahlfors SP,Ilmoniemi RJ,Hamalainen MS ( 1992): Estimates of visually evoked cortical currents. Electroencephalogr Clin Neurophysiol 82: 225–236. - PubMed
-
- Aine CJ,Supek S,George JS,Ranken D,Lewine J,Sanders J,Best E,Tiee W,Flynn ER,Wood CC ( 1996): Retinotopic organization of human visual cortex: Departures from the classical model. Cereb Cortex 6: 354–361. - PubMed
-
- Aine C,Huang M,Stephen J,Christner R ( 2000): Multistart algorithms for MEG empirical data analysis reliably characterize locations and time courses of multiple sources. Neuroimage 12: 159–172. - PubMed
-
- Avidan G,Harel M,Hendler T,Ben‐Bashat D,Zohary E,Malach R ( 2002): Contrast sensitivity in human visual areas and its relationship to object recognition. J Neurophysiol 87: 3102–3116. - PubMed
-
- Barth DS,Di S ( 1991): Laminar excitability cycles in neocortex. J Neurophysiol 65: 891–898. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

