Ligand tuning in asymmetric hydrovinylation of 1,3-dienes: a stereoselective route to either steroid-C20 (S) or -C20 (R) derivatives
- PMID: 18570419
- PMCID: PMC2652850
- DOI: 10.1021/ja711475f
Ligand tuning in asymmetric hydrovinylation of 1,3-dienes: a stereoselective route to either steroid-C20 (S) or -C20 (R) derivatives
Abstract
1,3-Dienes derived from steroidal D-ring C 17-ketones undergo Ni(II)-catalyzed hydrovinylation to give 1,2- or 1,4-addition of ethylene. Using finely tuned phosphoramidite ligands, it is possible to synthesize either the C 20 ( R)- or ( S)-derivatives without mutual contamination. The proportion of the 1,4-adduct, which is also formed stereoselectively, can be minimized by optimizing the reaction conditions. Because the two alkenes in the resultant dienes have differing steric demands for many potential reactions, and are ideally juxtaposed for further D-ring functionalization, these intermediates could be useful for the preparation of biologically important compounds such as vitamin D analogs and various antitumor steroidal glycosides.
Figures

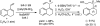






References
-
-
For an enantioselective synthesis and leading references, see: Watanabe H, Shimizu H, Mori K. Synthesis. 1994:1249..
-
-
-
For a review see, Zanoni G, Franzini M. Angew. Chem. Int. Ed. 2004;43:4837..
-
-
- Rowley M, Tsukamoto M, Kishi Y. J. Am. Chem. Soc. 1989;111:2735.
- Boeckman RK, Jr., Arvanitis A, Voss ME. J. Am. Chem. Soc. 1989;111:2737.
-
-
For a leading reference, Hijikuro I, Doi T, Takahashi T. J. Am. Chem. Soc. 2001;123:3716. For a review of vitamin D chemistry: Dai H, Posner GH. Synthesis. 1994:1383.Posner GH, Lee JK, White MC, Hutchings RH, Dai H, Kachinski JL, Dolan P, Kensler TW. J. Org. Chem. 1997;62:3299..
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

