Gold nanocages: synthesis, properties, and applications
- PMID: 18570442
- PMCID: PMC2645935
- DOI: 10.1021/ar800018v
Gold nanocages: synthesis, properties, and applications
Abstract
Noble-metal nanocages comprise a novel class of nanostructures possessing hollow interiors and porous walls. They are prepared using a remarkably simple galvanic replacement reaction between solutions containing metal precursor salts and Ag nanostructures prepared through polyol reduction. The electrochemical potential difference between the two species drives the reaction, with the reduced metal depositing on the surface of the Ag nanostructure. In our most studied example, involving HAuCl(4) as the metal precursor, the resultant Au is deposited epitaxially on the surface of the Ag nanocubes, adopting their underlying cubic form. Concurrent with this deposition, the interior Ag is oxidized and removed, together with alloying and dealloying, to produce hollow and, eventually, porous structures that we commonly refer to as Au nanocages. This approach is versatile, with a wide range of morphologies (e.g., nanorings, prism-shaped nanoboxes, nanotubes, and multiple-walled nanoshells or nanotubes) available upon changing the shape of the initial Ag template. In addition to Au-based structures, switching the metal salt precursors to Na(2)PtCl(4) and Na(2)PdCl(4) allows for the preparation of Pt- and Pd-containing hollow nanostructures, respectively. We have found that changing the amount of metal precursor added to the suspension of Ag nanocubes is a simple means of tuning both the composition and the localized surface plasmon resonance (LSPR) of the metal nanocages. Using this approach, we are developing structures for biomedical and catalytic applications. Because discrete dipole approximations predicted that the Au nanocages would have large absorption cross-sections and because their LSPR can be tuned into the near-infrared (where the attenuation of light by blood and soft tissue is greatly reduced), they are attractive materials for biomedical applications in which the selective absorption of light at great depths is desirable. For example, we have explored their use as contrast enhancement agents for both optical coherence tomography and photoacoustic tomography, with improved performance observed in each case. Because the Au nanocages have large absorption cross-sections, they are also effective photothermal transducers; thus, they might provide a therapeutic effect through selective hyperthermia-induced killing of targeted cancer cells. Our studies in vitro have illustrated the feasibility of applying this technique as a less-invasive form of cancer treatment.
Figures




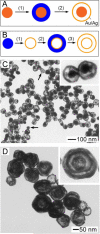
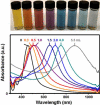

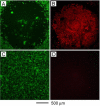

References
-
- Shipway AN, Katz E, Willner I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem. 2000;1:18–52. - PubMed
-
- Murphy CJ, San TK, Gole AM, Orendorff CJ, Gao JX, Gou L, Hunyadi SE, Li T. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J. Phys. Chem. B. 2005;109:13857–13870. - PubMed
-
- Willets KA, Van Duyne RP. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007;58:267–297. - PubMed
-
- Aiken JD, Finke RG. A review of modern transition-metal nanoclusters: their synthesis, characterization, and applications in catalysis. J. Mol. Catal. A-Chem. 1999;145:1–44.
-
- Murray CB, Kagan CR, Bawendi MG. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci. 2000;30:545–610.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

