Modified cell cycle status in a mouse model of altered neuronal vulnerability (slow Wallerian degeneration; Wlds)
- PMID: 18570652
- PMCID: PMC2481432
- DOI: 10.1186/gb-2008-9-6-r101
Modified cell cycle status in a mouse model of altered neuronal vulnerability (slow Wallerian degeneration; Wlds)
Abstract
Background: Altered neuronal vulnerability underlies many diseases of the human nervous system, resulting in degeneration and loss of neurons. The neuroprotective slow Wallerian degeneration (Wlds) mutation delays degeneration in axonal and synaptic compartments of neurons following a wide range of traumatic and disease-inducing stimuli, providing a powerful experimental tool with which to investigate modulation of neuronal vulnerability. Although the mechanisms through which Wlds confers neuroprotection remain unclear, a diverse range of downstream modifications, incorporating several genes/pathways, have been implicated. These include the following: elevated nicotinamide adenine dinucleotide (NAD) levels associated with nicotinamide mononucleotide adenylyltransferase 1 (Nmnat1; a part of the chimeric Wlds gene); altered mRNA expression levels of genes such as pituitary tumor transforming gene 1 (Pttg1); changes in the location/activity of the ubiquitin-proteasome machinery via binding to valosin-containing protein (VCP/p97); and modified synaptic expression of proteins such as ubiquitin-activating enzyme E1 (Ube1).
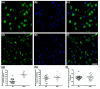
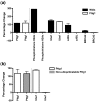
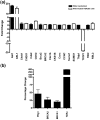
Results: Wlds expression in mouse cerebellum and HEK293 cells induced robust increases in a broad spectrum of cell cycle-related genes. Both NAD-dependent and Pttg1-dependent pathways were responsible for mediating different subsets of these alterations, also incorporating changes in VCP/p97 localization and Ube1 expression. Cell proliferation rates were not modified by Wlds, suggesting that later mitotic phases of the cell cycle remained unaltered. We also demonstrate that Wlds concurrently altered endogenous cell stress pathways.
Conclusion: We report a novel cellular phenotype in cells with altered neuronal vulnerability. We show that previous reports of diverse changes occurring downstream from Wlds expression converge upon modifications in cell cycle status. These data suggest a strong correlation between modified cell cycle pathways and altered vulnerability of axonal and synaptic compartments in postmitotic, terminally differentiated neurons.
Figures




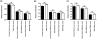




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

