Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids
- PMID: 18571270
- PMCID: PMC2532851
- DOI: 10.1016/j.jhep.2008.04.023
Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids
Abstract
Background/aims: We reported that reduced dietary intake of polyunsaturated fatty acids (PUFA) such as arachidonic (AA,20:4n6,omega-6) and docosahexaenoic (DHA,22:6n3,omega-3) acids led to alcohol-induced fatty liver and fibrosis. This study was aimed at studying the mechanisms by which a DHA/AA-supplemented diet prevents alcohol-induced fatty liver.
Methods: Male Long-Evans rats were fed an ethanol or control liquid-diet with or without DHA/AA for 9 weeks. Plasma transaminase levels, liver histology, oxidative/nitrosative stress markers, and activities of oxidatively-modified mitochondrial proteins were evaluated.
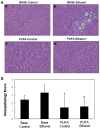
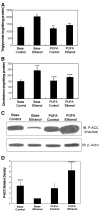
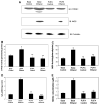
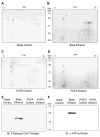
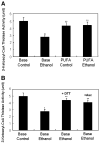
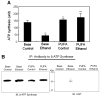
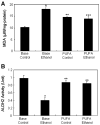
Results: Chronic alcohol administration increased the degree of fatty liver but fatty liver decreased significantly in rats fed the alcohol-DHA/AA-supplemented diet. Alcohol exposure increased oxidative/nitrosative stress with elevated levels of ethanol-inducible CYP2E1, nitric oxide synthase, nitrite and mitochondrial hydrogen peroxide. However, these increments were normalized in rats fed the alcohol-DHA/AA-supplemented diet. The number of oxidatively-modified mitochondrial proteins was markedly increased following alcohol exposure but significantly reduced in rats fed the alcohol-DHA/AA-supplemented diet. The suppressed activities of mitochondrial aldehyde dehydrogenase, ATP synthase, and 3-ketoacyl-CoA thiolase in ethanol-exposed rats were also recovered in animals fed the ethanol-DHA/AA-supplemented diet.
Conclusions: Addition of DHA/AA prevents alcohol-induced fatty liver and mitochondrial dysfunction in an animal model by protecting various mitochondrial enzymes most likely through reducing oxidative/nitrosative stress.
Figures
References
-
- Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006;1:420–439. - PubMed
-
- Schaefer EJ, Lichtenstein AH, Lamon-Fava S, McNamara JR, Schaefer MM, Rasmussen H, et al. Body weight and low-density lipoprotein cholesterol changes after consumption of a low-fat ad libitum diet. JAMA. 1995;274:1450–1455. - PubMed
-
- Salem N, Jr, Reyzer M, Karanian J. Losses of arachidonic acid in rat liver after alcohol inhalation. Lipids. 1996;31:S153–S156. - PubMed
-
- Salem N, Jr, Olsson NU. Abnormalities in essential fatty acid status in alcoholism. In: Yehuda S, Mostofsky DI, editors. Handbook of Essential Fatty Acid Biology: Biochemistry, Physiology and Behavioral Neurobiology. Totowa: Humana Press; 1997. pp. 67–87.
-
- Joly J-G, Hetu C. Effects of chronic ethanol administration in the rat: relative dependency on dietary lipids - I. Induction of hepatic drug-metabolizing enzymes in vitro. Biochem Pharmacol. 1975;24:1475–1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

