Zinc regulation of aminopeptidase B involved in neuropeptide production
- PMID: 18571504
- PMCID: PMC2764277
- DOI: 10.1016/j.febslet.2008.06.017
Zinc regulation of aminopeptidase B involved in neuropeptide production
Abstract
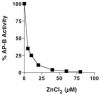
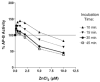
Aminopeptidase B (AP-B) is a metallopeptidase that removes basic residues from the N-termini of neuropeptide substrates in secretory vesicles. This study assessed zinc regulation of AP-B activity, since secretory vesicles contain endogenous zinc. AP-B was inhibited by zinc at concentrations typically present in secretory vesicles. Zinc effects were dependent on concentration, incubation time, and the molar ratio of zinc to enzyme. AP-B activity was recovered upon removal of zinc. AP-B with zinc became susceptible to degradation by trypsin, suggesting that zinc alters enzyme conformation. Zinc regulation demonstrates the metallopeptidase property of AP-B.
Figures




Similar articles
-
Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules.J Neurochem. 2007 Mar;100(5):1340-50. doi: 10.1111/j.1471-4159.2006.04325.x. Epub 2007 Jan 11. J Neurochem. 2007. PMID: 17241125
-
Aminopeptidase B, a glucagon-processing enzyme: site directed mutagenesis of the Zn2+-binding motif and molecular modelling.BMC Biochem. 2007 Oct 31;8:21. doi: 10.1186/1471-2091-8-21. BMC Biochem. 2007. PMID: 17974014 Free PMC article.
-
Proteolytic fragmentation reveals the oligomeric and domain structure of porcine aminopeptidase A.Biochemistry. 1997 Mar 11;36(10):3000-7. doi: 10.1021/bi962401q. Biochemistry. 1997. PMID: 9062131
-
The characteristics, functions and inhibitors of three aminopeptidases belonging to the m1 family.Curr Protein Pept Sci. 2012 Aug;13(5):490-500. doi: 10.2174/138920312802430554. Curr Protein Pept Sci. 2012. PMID: 22954453 Review.
-
Dipeptidyl peptidase III: a multifaceted oligopeptide N-end cutter.FEBS J. 2011 Sep;278(18):3256-76. doi: 10.1111/j.1742-4658.2011.08275.x. FEBS J. 2011. PMID: 21794094 Review.
Cited by
-
Characterization of a serine protease homologous to house dust mite group 3 allergens from the scabies mite Sarcoptes scabiei.J Biol Chem. 2009 Dec 4;284(49):34413-22. doi: 10.1074/jbc.M109.061911. Epub 2009 Oct 7. J Biol Chem. 2009. PMID: 19812030 Free PMC article.
-
A Combined N-terminomics and Shotgun Proteomics Approach to Investigate the Responses of Human Cells to Rapamycin and Zinc at the Mitochondrial Level.Mol Cell Proteomics. 2019 Jun;18(6):1085-1095. doi: 10.1074/mcp.RA118.001269. Epub 2019 Mar 15. Mol Cell Proteomics. 2019. PMID: 31154437 Free PMC article.
-
Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication.J Proteome Res. 2010 Oct 1;9(10):5002-24. doi: 10.1021/pr1003104. J Proteome Res. 2010. PMID: 20695487 Free PMC article.
-
Functional interaction of phosphatase and tensin homologue (PTEN) with the E3 ligase NEDD4-1 during neuronal response to zinc.J Biol Chem. 2010 Mar 26;285(13):9847-9857. doi: 10.1074/jbc.M109.091637. Epub 2010 Jan 25. J Biol Chem. 2010. PMID: 20100827 Free PMC article.
-
Human brain gene expression profiles of the cathepsin V and cathepsin L cysteine proteases, with the PC1/3 and PC2 serine proteases, involved in neuropeptide production.Heliyon. 2018 Jul 3;4(7):e00673. doi: 10.1016/j.heliyon.2018.e00673. eCollection 2018 Jul. Heliyon. 2018. PMID: 29998195 Free PMC article.
References
-
- Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook V. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–9595. - PMC - PubMed
-
- Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook V. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J Biol Chem. 2007;282:9556–9563. - PubMed
-
- Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745–20748. - PubMed
-
- Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol, and extracellular milieu. Essays Biochem. 2002;38:79–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

