Differential activation of multiple signalling pathways dictates eNOS upregulation by FGF2 but not VEGF in placental artery endothelial cells
- PMID: 18571718
- PMCID: PMC2596925
- DOI: 10.1016/j.placenta.2008.05.005
Differential activation of multiple signalling pathways dictates eNOS upregulation by FGF2 but not VEGF in placental artery endothelial cells
Abstract
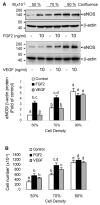
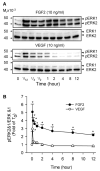
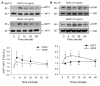
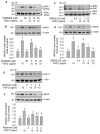
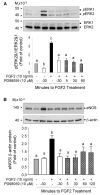
Fibroblast growth factor (FGF2), but not vascular endothelial growth factor (VEGF), upregulates endothelial nitric oxide synthase (eNOS) protein expression, at least partially, via activation of extracellular signal-regulated kinase 2/1 (ERK2/1) in ovine fetoplacental artery endothelial (oFPAE) cells. Herein we further investigated the temporal effects of FGF2 and VEGF on other signalling pathways including members (Jun N-terminal kinase JNK1/2 and p38MAPK) of mitogen-activated protein kinases (MAPK), phosphatidylinositol-3 kinase/v-akt murine thymoma viral oncogene homologue 1 (PI3K/AKT1), and the tyrosine kinase c-SRC, and examined if either one or more of these pathways play a role in the differential regulation of eNOS by FGF2 and VEGF. We first confirmed that in oFPAE cells, FGF2, but not VEGF, increased eNOS protein. FGF2 stimulated eNOS protein in a time- and concentration-dependent manner, which also depended on cell density. FGF2 provoked sustained (5min to 12h) whereas VEGF only stimulated transient (5min) ERK2/1 phosphorylation. FGF2 was 1.7-fold more potent in stimulating ERK2/1 phosphorylation than VEGF. FGF2 and VEGF only transiently activated JNK1/2 and AKT1 within 5min; however, FGF2 was a stronger stimulus than VEGF. FGF2 and VEGF did not significantly activate p38MAPK at 5min; however, VEGF stimulated p38MAPK phosphorylation at 60min. VEGF but not FGF2 significantly stimulated c-SRC phosphorylation. Inhibitors of MEK-ERK2/1 (PD98059), JNK1/2 (SP600125) and PI3K (wortmannin), but not p38MAPK (SB203580) and SRC (PP2), decreased the FGF2-increased eNOS protein expression. Thus, the FGF2-induced eNOS protein expression requires activation of multiple signalling pathways including ERK2/1, JNK1/2 and PI3K/AKT1. Differences in intensity and temporal patterns of activation of these pathways by FGF2 and VEGF may account for their differential effects on eNOS expression in OFPAE cells.
Figures

Similar articles
-
Deciphering mechanisms controlling placental artery endothelial cell migration stimulated by vascular endothelial growth factor.Endocrinology. 2010 Jul;151(7):3432-44. doi: 10.1210/en.2009-1305. Epub 2010 May 12. Endocrinology. 2010. PMID: 20463056 Free PMC article.
-
Protein phosphatase 3 differentially modulates vascular endothelial growth factor- and fibroblast growth factor 2-stimulated cell proliferation and signaling in ovine fetoplacental artery endothelial cells.Biol Reprod. 2008 Oct;79(4):704-10. doi: 10.1095/biolreprod.108.068957. Epub 2008 May 28. Biol Reprod. 2008. PMID: 18509162 Free PMC article.
-
Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation.Biol Reprod. 2008 Jan;78(1):143-50. doi: 10.1095/biolreprod.107.064477. Epub 2007 Sep 26. Biol Reprod. 2008. PMID: 17901071 Free PMC article.
-
Suppression of protein phosphatase 2 differentially modulates VEGF- and FGF2-induced signaling in ovine fetoplacental artery endothelial cells.Placenta. 2009 Oct;30(10):907-13. doi: 10.1016/j.placenta.2009.07.003. Epub 2009 Aug 18. Placenta. 2009. PMID: 19692121 Free PMC article.
-
Signaling regulation of fetoplacental angiogenesis.J Endocrinol. 2012 Mar;212(3):243-55. doi: 10.1530/JOE-11-0296. Epub 2011 Nov 21. J Endocrinol. 2012. PMID: 22106098 Free PMC article. Review.
Cited by
-
Activation of AP-1 transcription factors differentiates FGF2 and vascular endothelial growth factor regulation of endothelial nitric-oxide synthase expression in placental artery endothelial cells.J Biol Chem. 2010 Jun 4;285(23):17348-58. doi: 10.1074/jbc.M109.092791. Epub 2010 Apr 6. J Biol Chem. 2010. PMID: 20371606 Free PMC article.
-
Vascular Distribution and Expression Patterns of Angiogenic Factors in Caruncle during the Early Stage of Pregnancy in Goats (Capra hircus).Animals (Basel). 2022 Dec 27;13(1):99. doi: 10.3390/ani13010099. Animals (Basel). 2022. PMID: 36611709 Free PMC article.
-
Placental surface area mediates the association between FGFR2 methylation in placenta and full-term low birth weight in girls.Clin Epigenetics. 2018 Mar 22;10:39. doi: 10.1186/s13148-018-0472-5. eCollection 2018. Clin Epigenetics. 2018. PMID: 29588807 Free PMC article.
-
Rapid stromal remodeling by short-term VEGFR2 inhibition increases chemotherapy delivery in esophagogastric adenocarcinoma.Mol Oncol. 2020 Apr;14(4):704-720. doi: 10.1002/1878-0261.12599. Epub 2020 Mar 3. Mol Oncol. 2020. PMID: 31733011 Free PMC article.
-
Rac1-dependent intracellular superoxide formation mediates vascular endothelial growth factor-induced placental angiogenesis in vitro.Endocrinology. 2010 Nov;151(11):5315-25. doi: 10.1210/en.2010-0178. Epub 2010 Sep 15. Endocrinology. 2010. PMID: 20844008 Free PMC article.
References
-
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod. 2001;64:1033–40. - PubMed
-
- Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. Molecular Regulation Placenta. 2004;25:103–13. - PubMed
-
- Magness RR, Zheng J. Maternal cardiovascular alterations during pregnancy. In: Gluckman PD, Hermann MA, editors. Pediatric and Perinatal Perspectives. London: Edward Arnold Publishers; 1996. pp. 762–72.
-
- Bromwich PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Placental growth throughout the last two thirds of pregnancy in sheep: vascular development and angiogenic factor expression. Biol Reprod. 2007;76:259–67. - PubMed
-
- Choi JW, Im MW, Pai SH. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann Clin Lab Sci. 2002;32:257–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

