The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting
- PMID: 18571732
- PMCID: PMC2567873
- DOI: 10.1016/j.jneumeth.2008.05.003
The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting
Abstract
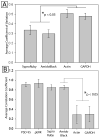
Western blots are used to estimate the relative concentrations of proteins of interest based on staining by specific antibodies. Quantitative measurements are often subject to error due to overloading of the loading control and over-reliance on normalization. We have found that at the protein concentrations normally used to quantify most low-abundance proteins of interest, frequently used single-protein loading controls, such as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and beta-actin, do not accurately reflect differences in protein concentration. Two total protein stains, SYPRO Ruby and Amido Black, were compared and found to be acceptable alternatives to single-protein controls. Although we cannot prove that high-abundance loading controls are inaccurate under all possible conditions, we conclude that the burden of proof should lie with the researcher to demonstrate that their loading control is reflective of quantitative differences in protein concentration.
Figures


References
-
- Alexander M, Curtis G, Avruch J, Goodman HM. Insulin regulation of protein biosynthesis in differentiated 3T3 adipocytes. Regulation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1985;260:11978–85. - PubMed
-
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–27. - PubMed
-
- Dittmer A, Dittmer J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27:2844–5. - PubMed
-
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes and immunity. 2005;6:279–84. - PubMed
-
- Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;19:1837–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

