Molecular basis of oncostatin M-induced SOCS-3 expression in astrocytes
- PMID: 18571793
- PMCID: PMC2621074
- DOI: 10.1002/glia.20694
Molecular basis of oncostatin M-induced SOCS-3 expression in astrocytes
Abstract
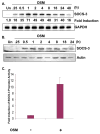
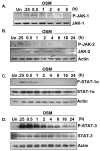
Under neuropathological conditions, reactive astrocytes release cytokines and chemokines, which act in an autocrine and/or paracrine fashion to modulate production of immunoregulatory factors from cells including microglia, astrocytes, and neurons. In this way, astrocytes play an important role in orchestrating immune responses within the central nervous system (CNS). Suppressor of cytokine signaling (SOCS) proteins are endogenous, negative regulators of the JAK/STAT signaling pathway and function as attenuators of the immune and inflammatory responses. As such, SOCS proteins may have critical roles in the CNS under neuroinflammatory conditions. In the inflamed CNS, expression of IL-6 cytokine family member oncostatin M (OSM) is elevated; however, its functional effects are not well understood. We demonstrate that OSM is a potent inducer of SOCS-3 in astrocytes. Analysis of the SOCS-3 promoter revealed that an AP-1 element, two IFN-gamma activation sequence (GAS) elements, and a GC-rich region are crucial for SOCS-3 gene expression. Using small interfering RNA against STAT-3, as well as a STAT-3 dominant-negative construct, we demonstrate that STAT-3 activation is critical for OSM induction of SOCS-3 expression. The ERK1/2 and JNK pathways also contribute to OSM-induced SOCS-3 gene expression. OSM stimulation led to a time-dependent recruitment of the transcription factors STAT-3, c-Fos, c-Jun, and Sp1 and the coactivators CREB-binding protein (CBP) and p300 to the endogenous SOCS-3 promoter. These data indicate that OSM-induced activation of STAT-3 and the ERK1/2 and JNK pathways are critical for astrocytic expression of SOCS-3, which provides for feedback inhibition of cytokine-induced inflammatory responses in the CNS.
(c) 2008 Wiley-Liss, Inc.
Figures







References
-
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. - PubMed
-
- Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem. 2004;279:54708–54715. - PubMed
-
- Barclay JL, Anderson ST, Waters MJ, Curlewis JD. Characterization of the SOCS3 promoter response to prostaglandin E2 in T47D cells. Mol Endocrinol. 2007a;21:2516–2528. - PubMed
-
- Barclay JL, Anderson ST, Waters MJ, Curlewis JD. Regulation of suppressor of cytokine signaling 3 (SOC3) by growth hormone in pro-B cells. Mol Endocrinol. 2007b;21:2503–2515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

