Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice
- PMID: 18571864
- PMCID: PMC2664643
- DOI: 10.1016/j.neuroscience.2008.05.017
Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice
Abstract
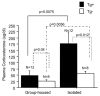
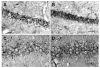
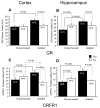
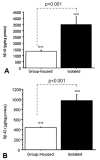
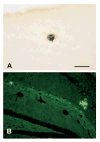
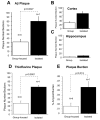
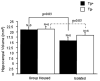
Previously, we reported that the stress associated with chronic isolation was associated with increased beta-amyloid (Abeta) plaque deposition and memory deficits in the Tg2576 transgenic animal model of Alzheimer's disease (AD) [Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG (2004) Effects of isolation stress on hippocampal neurogenesis, memory, and amyloid plaque deposition in APP (Tg2576) mutant mice. Neuroscience 127:601-609]. In this study, we investigated the potential mechanisms of stress-accelerated Abeta plaque deposition in this Tg2576 mice by examining the relationship between plasma corticosterone levels, expression of glucocorticoid receptor (GR) and corticotropin-releasing factor receptor-1 (CRFR1) in the brain, brain tissue Abeta levels and Abeta plaque deposition during isolation or group housing from weaning (i.e. 3 weeks of age) until 27 weeks of age. We found that isolation housing significantly increased plasma corticosterone levels as compared with group-housing in both Tg+ mice (which contain and overexpress human amyloid precursor protein (hAPP) gene) and Tg- mice (which do not contain hAPP gene as control). Also, isolated, but not group-housed animals showed increases in the expression of GR in the cortex. Furthermore, the expression of CRFR1 was increased in isolated Tg+ mice, but decreased in isolated Tg- mice in both cortex and hippocampus. Changes in the components of hypothalamic-pituitary-adrenal (HPA) axis were accompanied by increases in brain tissue Abeta levels and Abeta plaque deposition in the hippocampus and overlying cortex in isolated Tg+ mice. These results suggest that isolation stress increases corticosterone levels and GR and CRFR1 expression in conjunction with increases in brain tissue Abeta levels and Abeta plaque deposition in the Tg2576 mouse model of AD.
Figures
References
-
- Alfarez DN, Joëls M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topological and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. - PubMed
-
- Auchus AP, Green RC, Nemeroff CB. Cortical and subcortical neuropeptides in Alzheimer's disease. Neurobiol Aging. 1994;15:589–595. - PubMed
-
- Behan DP, Khongsaly O, Owens MJ, Chung HD, Nemeroff CB, De Souza EB. Corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer's disease and control postmortem human brain. J Neurochem. 1997;68:2053–2060. - PubMed
-
- Blank T, Nijholt I, Vollstaedt S, Spiess J. The corticotropin-releasing factor receptor 1 antagonist CP-154,526 reverses stress-induced learning deficits in mice. Behav Brain Res. 2003;138:207–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

