Vasopressin up-regulates the expression of growth-related immediate-early genes via two distinct EGF receptor transactivation pathways
- PMID: 18571897
- PMCID: PMC2602840
- DOI: 10.1016/j.cellsig.2008.05.009
Vasopressin up-regulates the expression of growth-related immediate-early genes via two distinct EGF receptor transactivation pathways
Abstract
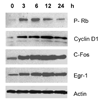
Activation of V(1a) receptor triggers the expression of growth-related immediate-early genes (IEGs), including c-Fos and Egr-1. We found that pre-treatment of rat vascular smooth muscle A-10 cell line with the EGF receptor inhibitor AG1478 or the over-expression of an EGFR dominant negative mutant (HEBCD533) blocked the vasopressin-induced expression of IEGs, suggesting that activation of these early genes mediated by V(1a) receptor is via transactivation of the EGF receptor. Importantly, the inhibition of the metalloproteinases, which catalyzed the shedding of the EGF receptor agonist HB-EGF, selectively blocked the vasopressin-induced expression c-Fos. On the other hand, the inhibition of c-Src selectively blocked the vasopressin-induced expression of Egr-1. Interestingly, in contrast to the expression of c-Fos, the expression of Egr-1 was mediated via the Ras/MEK/MAPK-dependent signalling pathway. Vasopressin-triggered expression of both genes required the release of intracellular calcium, activation of PKC and beta-arrestin 2. These findings demonstrated that vasopressin up-regulated the expression of c-Fos and Erg-1 via transactivation of two distinct EGF receptor-dependent signalling pathways.
Figures





Similar articles
-
Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor.J Biol Chem. 2009 May 1;284(18):11953-62. doi: 10.1074/jbc.M808176200. Epub 2009 Mar 2. J Biol Chem. 2009. PMID: 19254952 Free PMC article.
-
beta-Migrating very low density lipoprotein (beta VLDL) activates smooth muscle cell mitogen-activated protein (MAP) kinase via G protein-coupled receptor-mediated transactivation of the epidermal growth factor (EGF) receptor: effect of MAP kinase activation on beta VLDL plus EGF-induced cell proliferation.J Biol Chem. 2001 Aug 17;276(33):30579-88. doi: 10.1074/jbc.M103761200. Epub 2001 May 25. J Biol Chem. 2001. PMID: 11375998
-
Arginine vasopressin enhances cell survival via a G protein-coupled receptor kinase 2/β-arrestin1/extracellular-regulated kinase 1/2-dependent pathway in H9c2 cells.Mol Pharmacol. 2013 Aug;84(2):227-35. doi: 10.1124/mol.113.086322. Epub 2013 May 20. Mol Pharmacol. 2013. PMID: 23690069 Free PMC article.
-
Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation.Circ Res. 2001 Jan 19;88(1):22-9. doi: 10.1161/01.res.88.1.22. Circ Res. 2001. Retraction in: Circ Res. 2013 Jun 7;112(12):e180. doi: 10.1161/RES.0b013e31829b5cca. PMID: 11139469 Retracted.
-
beta-Arrestin 2 expression determines the transcriptional response to lysophosphatidic acid stimulation in murine embryo fibroblasts.J Biol Chem. 2005 Sep 16;280(37):32157-67. doi: 10.1074/jbc.M507460200. Epub 2005 Jul 15. J Biol Chem. 2005. PMID: 16027114
Cited by
-
BRET-based assay to monitor EGFR transactivation by the AT1R reveals Gq/11 protein-independent activation and AT1R-EGFR complexes.Biochem Pharmacol. 2018 Dec;158:232-242. doi: 10.1016/j.bcp.2018.10.017. Epub 2018 Oct 19. Biochem Pharmacol. 2018. PMID: 30347205 Free PMC article.
-
beta-Arrestin mediates beta1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling.J Biol Chem. 2009 Jul 24;284(30):20375-86. doi: 10.1074/jbc.M109.005793. Epub 2009 Jun 9. J Biol Chem. 2009. PMID: 19509284 Free PMC article.
-
GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness.PLoS One. 2011;6(6):e21599. doi: 10.1371/journal.pone.0021599. Epub 2011 Jun 28. PLoS One. 2011. PMID: 21738726 Free PMC article.
-
Constitutive coupling of a naturally occurring human alpha1a-adrenergic receptor genetic variant to EGFR transactivation pathway.Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19796-801. doi: 10.1073/pnas.1116271108. Epub 2011 Nov 16. Proc Natl Acad Sci U S A. 2011. PMID: 22089237 Free PMC article.
-
IL-1beta signals through the EGF receptor and activates Egr-1 through MMP-ADAM.PLoS One. 2012;7(7):e39811. doi: 10.1371/journal.pone.0039811. Epub 2012 Jul 6. PLoS One. 2012. PMID: 22792188 Free PMC article.
References
-
- Gonzalez CB, Figueroa CD. Biol Res. 1999;32:63–76. - PubMed
-
- Sarmiento JM, Ehrenfeld P, Anazco CC, Reyes CE, Troncoso S, Figueroa CD, Muller-Esterl W, Gonzalez CB. Kidney Int. 2005;68:487–496. - PubMed
-
- Kreisberg JI, Venkatachalam M, Troyer D. Am J Physiol. 1985;249:F457–F463. - PubMed
-
- Segarra G, Medina P, Vila JM, Chuan P, Domenech C, Lluch S. J Hypertens. 2002;20:1373–1379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

