Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial
- PMID: 18572080
- PMCID: PMC2650104
- DOI: 10.1016/S0140-6736(08)60920-4
Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial
Abstract
Background: Across many observational studies, herpes simplex virus type 2 (HSV-2) infection is associated with two-fold to three-fold increased risk for HIV-1 infection. We investigated whether HSV-2 suppression with aciclovir would reduce the risk of HIV-1 acquisition.
Methods: We undertook a double-blind, randomised, placebo-controlled phase III trial in HIV-negative, HSV-2 seropositive women in Africa and men who have sex with men (MSM) from sites in Peru and the USA. Participants were randomly assigned by block randomisation to twice daily aciclovir 400 mg (n=1637) or matching placebo (n=1640) for 12-18 months, and were seen monthly for dispensation of study drug, adherence counselling and measurement by pill count and self-reporting, and risk reduction counselling, and every 3 months for genital examination and HIV testing. The primary outcome was HIV-1 acquisition and secondary was incidence of genital ulcers. Analysis was by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00076232.
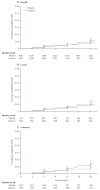
Findings: 3172 participants (1358 women, 1814 MSM) were included in the primary dataset (1581 in aciclovir group, 1591 in control group). The incidence of HIV-1 was 3.9 per 100 person-years in the aciclovir group (75 events in 1935 person-years of follow-up) and 3.3 per 100 person-years in the placebo group (64 events in 1969 person-years of follow-up; hazard ratio 1.16 [95% CI 0.83-1.62]). Incidence of genital ulcers on examination was reduced by 47% (relative risk 0.53 [0.46-0.62]) and HSV-2 positive genital ulcers by 63% (0.37 [0.31-0.45]) in the aciclovir group. Adherence to dispensed study drug was 94% in the aciclovir group and 94% in the placebo group, and 85% of expected doses in the aciclovir group and 86% in the placebo group. Retention was 85% at 18 months in both groups (1028 of 1212 in aciclovir group, 1030 of 1208 in placebo group). We recorded no serious events related to the study drug.
Interpretation: Our results show that suppressive therapy with standard doses of aciclovir is not effective in reduction of HIV-1 acquisition in HSV-2 seropositive women and MSM. Novel strategies are needed to interrupt interactions between HSV-2 and HIV-1.
Conflict of interest statement
Figures
Comment in
-
Reassessing the hypothesis on STI control for HIV prevention.Lancet. 2008 Jun 21;371(9630):2064-5. doi: 10.1016/S0140-6736(08)60896-X. Lancet. 2008. PMID: 18572064 No abstract available.
-
Effect of aciclovir on HIV-1 acquisition in HSV-2-positive patients.Lancet. 2008 Oct 11;372(9646):1298; author reply 1298-9. doi: 10.1016/S0140-6736(08)61543-3. Lancet. 2008. PMID: 18929897 No abstract available.
References
-
- Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(suppl 1):24A–35A. - PubMed
-
- Lama JR, Lucchetti A, Suarez L, et al. Association of herpes simplex virus type 2 infection and syphilis with human immunodeficiency virus infection among men who have sex with men in Peru. J Infect Dis. 2006;194:1459–66. - PubMed
-
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–50. - PubMed
-
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. - PubMed
-
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

