Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils
- PMID: 18572241
- PMCID: PMC2597220
- DOI: 10.1016/j.ceca.2008.05.001
Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils
Abstract
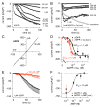
The Ca(2+)-permeable TRPM2 channel is a dual function protein that is activated by intracellular ADPR through its enzymatic pyrophosphatase domain with Ca(2+) acting as a co-factor. Other TRPM2 regulators include cADPR, NAADP and H(2)O(2), which synergize with ADPR to potentiate TRPM2 activation. Although TRPM2 has been thoroughly characterized in overexpression or cell-line systems, little is known about the features of TRPM2 in primary cells. We here characterize the regulation of TRPM2 activation in human neutrophils and report that ADPR activates TRPM2 with an effective half-maximal concentration (EC(50)) of 1microM. Potentiation by Ca(2+) is dose-dependent with an EC(50) of 300nM. Both cADPR and NAADP activate TRPM2, albeit with lower efficacy than in the presence of subthreshold levels of ADPR (100nM), which significantly shifts the EC(50) for cADPR from 44 to 3muM and for NAADP from 95 to 1microM. TRPM2 activation by ADPR can be suppressed by AMP with an IC(50) of 10microM and cADPR-induced activation can be blocked by 8-Bromo-cADPR. We further show that 100microM H(2)O(2) enables subthreshold concentrations of ADPR (100nM) to activate TRPM2. We conclude that agonistic and antagonistic characteristics of TRPM2 as seen in overexpression systems are largely compatible with the functional properties of TRPM2 currents measured in human neutrophils, but the potencies of agonists in primary cells are significantly higher.
Figures



References
-
- Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–9. - PubMed
-
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–73. - PubMed
-
- Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:307–14. - PubMed
-
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–9. - PubMed
-
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

