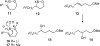Oxygen-directed intramolecular hydroboration
- PMID: 18572941
- PMCID: PMC2646882
- DOI: 10.1021/ja800402g
Oxygen-directed intramolecular hydroboration
Abstract
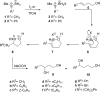
Metal-free homoallylic oxygen-directed intramolecular hydroboration is reported. Regioselectivities from 20:1 to 82:1 favoring the 1,3-dioxy-substituted products have been achieved using Me2S.BH3/TfOH followed by standard oxidative workup. Branching at the C5 position improves regioselectivity.
References
-
- Hoveyda A. H.; Evans D. A.; Fu G. C. Chem. Rev. 1993, 93, 1307.
-
- Evans D. A.; Muci A. R.; Stürmer R. J. Org. Chem. 1993, 58, 5307.
- Evans D. A.; Fu G. C.; Hoveyda A. H. J. Am. Chem. Soc. 1988, 110, 6917.
- Evans D. A.; Fu G. J. Am. Chem. Soc. 1991, 113, 4042.
-
- Panek J. S.; Xu F. J. Org. Chem. 1992, 25, 215.
-
- Schulte-Elte K. H.; Ohloff G. Helv. Chim. Acta 1967, 50, 153. - PubMed
- Heathcock C. H.; Jarvi E. T.; Rosen T. Tetrahedron Lett. 1984, 25, 243.
- Smith A. B.; Yokoyama Y.; Huryn D. M.; Dunlap N. K. Tetrahedron Lett. 1987, 28, 3659.
- Zweifel G.; Najafi M. R.; Rajagopalan S. Tetrahedron Lett. 1988, 29, 1895.
- Suzuki K.; Miyazawa M.; Shimazaki M.; Tsuchihashi G. Tetrahedron 1988, 44, 4061.
- Jung M. E.; Karama U. Tetrahedron Lett. 1999, 40, 7907.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous