Development of the retinotectal system in the direct-developing frog Eleutherodactylus coqui in comparison with other anurans
- PMID: 18573199
- PMCID: PMC2442589
- DOI: 10.1186/1742-9994-5-9
Development of the retinotectal system in the direct-developing frog Eleutherodactylus coqui in comparison with other anurans
Abstract
Background: Frogs primitively have a biphasic life history with an aquatic larva (tadpole) and a usually terrestrial adult. However, direct developing frogs of the genus Eleutherodactylus have lost a free living larval stage. Many larval structures never form during development of Eleutherodactylus, while limbs, spinal cord, and an adult-like cranial musculoskeletal system develop precociously.
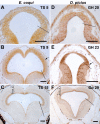
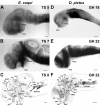
Results: Here, I compare growth and differentiation of the retina and tectum and development of early axon tracts in the brain between Eleutherodactylus coqui and the biphasically developing frogs Discoglossus pictus, Physalaemus pustulosus, and Xenopus laevis using morphometry, immunohistochemical detection of proliferating cell nuclear antigen (PCNA) and acetylated tubulin, biocytin tracing, and in situ hybridization for NeuroD. Findings of the present study indicate that retinotectal development was greatly altered during evolution of Eleutherodactlyus mostly due to acceleration of cell proliferation and growth in retina and tectum. However, differentiation of retina, tectum, and fiber tracts in the embryonic brain proceed along a conserved slower schedule and remain temporally coordinated with each other in E. coqui.
Conclusion: These findings reveal a mosaic pattern of changes in the development of the central nervous system (CNS) during evolution of the direct developing genus Eleutherodactylus. Whereas differentiation events in directly interconnected parts of the CNS such as retina, tectum, and brain tracts remained coordinated presumably due to their interdependent development, they were dissociated from proliferation control and from differentiation events in other parts of the CNS such as the spinal cord. This suggests that mosaic evolutionary changes reflect the modular character of CNS development.
Figures





Similar articles
-
Mosaic evolution of neural development in anurans: acceleration of spinal cord development in the direct developing frog Eleutherodactylus coqui.Anat Embryol (Berl). 2003 Feb;206(3):215-27. doi: 10.1007/s00429-002-0291-4. Epub 2003 Jan 21. Anat Embryol (Berl). 2003. PMID: 12592573
-
Development of the retina is altered in the directly developing frog Eleutherodactylus coqui (Leptodactylidae).Neurosci Lett. 1997 Mar 21;224(3):153-6. doi: 10.1016/S0304-3940(97)00174-2. Neurosci Lett. 1997. PMID: 9131659
-
Evolution of nerve development in frogs. II. Modified development of the peripheral nervous system in the direct-developing frog Eleutherodactylus coqui (Leptodactylidae).Brain Behav Evol. 1997;50(2):94-128. doi: 10.1159/000113325. Brain Behav Evol. 1997. PMID: 9261555
-
The big potential of the small frog Eleutherodactylus coqui.Elife. 2022 Jan 14;11:e73401. doi: 10.7554/eLife.73401. Elife. 2022. PMID: 35029143 Free PMC article. Review.
-
Frogs without polliwogs: evolution of anuran direct development.Bioessays. 2001 Mar;23(3):233-41. doi: 10.1002/1521-1878(200103)23:3<233::AID-BIES1033>3.0.CO;2-Q. Bioessays. 2001. PMID: 11223880 Review.
Cited by
-
Morphogenetic and Histogenetic Roles of the Temporal-Spatial Organization of Cell Proliferation in the Vertebrate Corticogenesis as Revealed by Inter-specific Analyses of the Optic Tectum Cortex Development.Front Cell Neurosci. 2016 Mar 17;10:67. doi: 10.3389/fncel.2016.00067. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27013978 Free PMC article. Review.
-
Developmental diversity of amphibians.Wiley Interdiscip Rev Dev Biol. 2012 May-Jun;1(3):345-69. doi: 10.1002/wdev.23. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 22662314 Free PMC article. Review.
-
Expression of cyclin D1, cyclin D2, and N-myc in embryos of the direct developing frog Eleutherodactylus coqui, with a focus on limbs.Gene Expr Patterns. 2013 Jun-Jul;13(5-6):142-9. doi: 10.1016/j.gep.2013.02.005. Epub 2013 Mar 7. Gene Expr Patterns. 2013. PMID: 23473789 Free PMC article.
-
The Use of Cognition by Amphibians Confronting Environmental Change: Examples from the Behavioral Ecology of Crawfish Frogs (Rana areolata).Animals (Basel). 2025 Mar 4;15(5):736. doi: 10.3390/ani15050736. Animals (Basel). 2025. PMID: 40076019 Free PMC article.
-
Characterization of the nutritional endoderm in the direct developing frog Eleutherodactylus coqui.Dev Genes Evol. 2013 Nov;223(6):351-62. doi: 10.1007/s00427-013-0451-y. Epub 2013 Sep 17. Dev Genes Evol. 2013. PMID: 24043604
References
-
- Dodd MHI, Dodd JM. The biology of metamorphosis. In: Lofts B, editor. Physiology of the amphibia III. New York: Academic Press; 1976. pp. 467–599.
-
- Szarski H. The origin of the larva and metamorphosis in amphibia. Am Nat. 1957;91:283–301.
-
- Wassersug RJ, Hoff K. Developmental changes in the orientation of the anuran jaw suspension. Evol Biol. 1982;15:223–246.
-
- Wake MH. Phylogenesis of direct development and viviparity in vertebrates. In: Wake DB, Roth G, editor. Complex organismal functions: integration and evolution in vertebrates. Chichester: Wiley; 1989. pp. 235–250.
LinkOut - more resources
Full Text Sources
Miscellaneous

