Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1
- PMID: 18573216
- PMCID: PMC2442059
- DOI: 10.1186/1749-8104-3-15
Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1
Abstract
Background: Beta-amyloid precursor protein (APP) has been reported to play a role in the outgrowth of neurites from cultured neurons. Both cell-surface APP and its soluble, ectodomain cleavage product (APPs-alpha) have been implicated in regulating the length and branching of neurites in a variety of assays, but the mechanism by which APP performs this function is not understood.
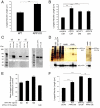
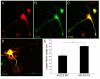
Results: Here, we report that APP is required for proper neurite outgrowth in a cell autonomous manner, both in vitro and in vivo. Neurons that lack APP undergo elongation of their longest neurite. Deletion of APLP1 or APLP2, homologues of APP, likewise stimulates neurite lengthening. Intriguingly, wild-type neurons exposed to APPs-alpha, the principal cleavage product of APP, also undergo neurite elongation. However, APPs-alpha is unable to stimulate neurite elongation in the absence of cellular APP expression. The outgrowth-enhancing effects of both APPs-alpha and the deletion of APP are inhibited by blocking antibodies to Integrin beta1 (Itgbeta1). Moreover, full length APP interacts biochemically with Itgbeta1, and APPs-alpha can interfere with this binding.
Conclusion: Our findings indicate that APPs-alpha regulates the function of APP in neurite outgrowth via the novel mechanism of competing with the binding of APP to Itgbeta1.
Figures



Similar articles
-
Interaction of reelin with amyloid precursor protein promotes neurite outgrowth.J Neurosci. 2009 Jun 10;29(23):7459-73. doi: 10.1523/JNEUROSCI.4872-08.2009. J Neurosci. 2009. PMID: 19515914 Free PMC article.
-
Cell-surface beta-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner.J Neurosci. 1995 Mar;15(3 Pt 2):2157-67. doi: 10.1523/JNEUROSCI.15-03-02157.1995. J Neurosci. 1995. PMID: 7891158 Free PMC article.
-
Secretion of nerve growth factor from septum stimulates neurite outgrowth and release of the amyloid protein precursor of Alzheimer's disease from hippocampal explants.J Neurosci Res. 1994 Jun 15;38(3):248-58. doi: 10.1002/jnr.490380303. J Neurosci Res. 1994. PMID: 7932862
-
APP independent and dependent effects on neurite outgrowth are modulated by the receptor associated protein (RAP).J Neurochem. 2013 Jan;124(1):123-32. doi: 10.1111/jnc.12051. Epub 2012 Nov 15. J Neurochem. 2013. PMID: 23061396 Free PMC article.
-
Amyloid precursor-like protein 1 (APLP1) exhibits stronger zinc-dependent neuronal adhesion than amyloid precursor protein and APLP2.J Neurochem. 2016 Apr;137(2):266-76. doi: 10.1111/jnc.13540. Epub 2016 Mar 7. J Neurochem. 2016. PMID: 26801522
Cited by
-
Physiological functions of APP family proteins.Cold Spring Harb Perspect Med. 2012 Feb;2(2):a006288. doi: 10.1101/cshperspect.a006288. Cold Spring Harb Perspect Med. 2012. PMID: 22355794 Free PMC article. Review.
-
Soluble SORLA Enhances Neurite Outgrowth and Regeneration through Activation of the EGF Receptor/ERK Signaling Axis.J Neurosci. 2020 Jul 29;40(31):5908-5921. doi: 10.1523/JNEUROSCI.0723-20.2020. Epub 2020 Jun 29. J Neurosci. 2020. PMID: 32601248 Free PMC article.
-
Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP.Proc Natl Acad Sci U S A. 2010 Oct 5;107(40):17362-7. doi: 10.1073/pnas.1012568107. Epub 2010 Sep 20. Proc Natl Acad Sci U S A. 2010. PMID: 20855613 Free PMC article.
-
Platelets and Alzheimer's disease: Potential of APP as a biomarker.World J Psychiatry. 2012 Dec 22;2(6):102-13. doi: 10.5498/wjp.v2.i6.102. World J Psychiatry. 2012. PMID: 24175176 Free PMC article. Review.
-
Identification of "sarsasapogenin-aglyconed" timosaponins as novel Aβ-lowering modulators of amyloid precursor protein processing.Chem Sci. 2016 May 1;7(5):3206-3214. doi: 10.1039/c5sc02377g. Epub 2016 Jan 22. Chem Sci. 2016. PMID: 29997812 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

