Modafinil decreases food intake in humans subjected to simulated shift work
- PMID: 18573275
- PMCID: PMC5858899
- DOI: 10.1016/j.pbb.2008.05.018
Modafinil decreases food intake in humans subjected to simulated shift work
Abstract
In a limited number of studies modafinil has been shown to decrease food intake by laboratory animals and humans. The present study represents a secondary data analysis, in which the effects of modafinil on several measures of food intake were determined in humans living in a residential laboratory during simulated shift work. During this 23-day study, a wide selection of food items and beverages were freely available. During this double-blind, within-participant study, volunteers (N = 11) received oral modafinil dose (0, 200, or 400 mg) 1 h after waking for three consecutive days under two shift conditions: day shift and night shift. Shifts alternated three times during the study, and shift conditions were separated by an "off" day. Modafinil (200, 400 mg) dose-dependently decreased total caloric intake by approximately 18% and approximately 38%, respectively, regardless of shift condition, without selectively altering the proportion of total calories derived from carbohydrate, fat and protein. Ratings of "Hungry" were also significantly decreased by both active doses, but only immediately before the lunch break period. In addition, tolerance to the anorexic effects of modafinil was not apparent, as these effects remained stable across the three days of modafinil dosing. These findings show that modafinil produced clear reductions in food intake and suggest that future prospective studies should examine the drug in obese participants.
Conflict of interest statement
Figures
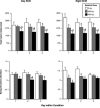
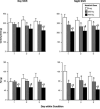
Similar articles
-
Modafinil attenuates disruptions in cognitive performance during simulated night-shift work.Neuropsychopharmacology. 2006 Jul;31(7):1526-36. doi: 10.1038/sj.npp.1300991. Epub 2005 Dec 14. Neuropsychopharmacology. 2006. PMID: 16395298 Clinical Trial.
-
Modafinil improves alertness, vigilance, and executive function during simulated night shifts.Sleep. 2004 May 1;27(3):434-9. doi: 10.1093/sleep/27.3.434. Sleep. 2004. PMID: 15164895 Clinical Trial.
-
Wake-promoting agents with different mechanisms of action: comparison of effects of modafinil and amphetamine on food intake and cardiovascular activity.Appetite. 2004 Apr;42(2):185-95. doi: 10.1016/j.appet.2003.11.003. Appetite. 2004. PMID: 15010183 Clinical Trial.
-
Modafinil in the treatment of excessive daytime sleepiness.Cleve Clin J Med. 2007 Aug;74(8):561-6, 568-71. doi: 10.3949/ccjm.74.8.561. Cleve Clin J Med. 2007. PMID: 17708127 Review.
-
Shift work sleep disorder: burden of illness and approaches to management.Drugs. 2006;66(18):2357-70. doi: 10.2165/00003495-200666180-00007. Drugs. 2006. PMID: 17181377 Review.
Cited by
-
Low striatal dopamine receptor availability linked to caloric intake during abstinence from chronic methamphetamine abuse.Mol Psychiatry. 2012 Jun;17(6):569-71. doi: 10.1038/mp.2011.137. Epub 2011 Oct 25. Mol Psychiatry. 2012. PMID: 22024765 Free PMC article. No abstract available.
-
Narcolepsy with cataplexy associated with nocturnal compulsive behaviors: a case-control study.Sleep. 2011 Oct 1;34(10):1365-71. doi: 10.5665/SLEEP.1280. Sleep. 2011. PMID: 21966068 Free PMC article.
-
Pitolisant, a wake-promoting agent devoid of psychostimulant properties: Preclinical comparison with amphetamine, modafinil, and solriamfetol.Pharmacol Res Perspect. 2021 Oct;9(5):e00855. doi: 10.1002/prp2.855. Pharmacol Res Perspect. 2021. PMID: 34423920 Free PMC article.
-
Depression-like deficits in rats improved by subchronic modafinil.Psychopharmacology (Berl). 2009 Jul;204(4):627-39. doi: 10.1007/s00213-009-1493-8. Epub 2009 Mar 3. Psychopharmacology (Berl). 2009. PMID: 19255746
-
A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans.Psychopharmacology (Berl). 2012 Jan;219(1):109-22. doi: 10.1007/s00213-011-2383-4. Epub 2011 Jun 30. Psychopharmacology (Berl). 2012. PMID: 21713605 Free PMC article.
References
-
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. - PubMed
-
- Biederman J, Swanson JM, Wigal SB, Kratochvil CJ, Boellner SW, Earl CQ, Jiang J, Greenhill L. Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics. 2005;116(6):e777–784. - PubMed
-
- Boellner SW, Earl CQ, Arora S. Modafinil in children and adolescents with attention-deficit/hyperactivity disorder: a preliminary 8-week, open-label study. Curr Med Res Opin. 2006;22(12):2457–2465. - PubMed
-
- Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27(6):1181–1194. - PubMed
-
- Broughton RJ, Fleming JA, George CF, Hill JD, Kryger MH, Moldofsky H, Montplaisir JY, Morehouse RL, Moscovitch A, Murphy WF. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology. 1997;49(2):444–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

